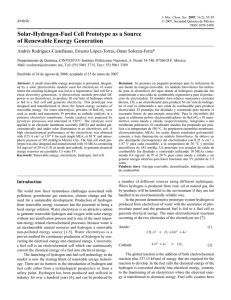
Computational and Theoretical Chemistry 1217 (2022) 113899 Contents lists available at ScienceDirect Computational and Theoretical Chemistry journal homepage: www.elsevier.com/locate/comptc Reversible hydrogen storage capacity of vanadium decorated small boron clusters (BnV2, n = 6–10): A dispersion corrected density functional study Shakti S Ray , Rakesh K Sahoo , Sridhar Sahu * Computational Material Research Laboratory, Department of Physics, Indian Institute of Technology (Indian School of Mines), Dhanbad, India A R T I C L E I N F O A B S T R A C T Keywords: Hydrogen storage Boron clusters DFT calculation QTAIM ADMP-MD simulation We present our theoretical investigation on hydrogen storage capacity of vanadium decorated small boron clusters (BnV2, n = 6–10) employing dispersion-corrected density functional theory. Stabilities of the clusters during H2 adsorption are confirmed from the global reactivity parameters. BnV2 clusters are found to adsorb up to ten H2 molecules in quasi-molecular form via Kubas-Niu-Rao-Jena kind of interactions with average adsorption energy in the range of 0.17–0.26 eV/H2. ADMP molecular dynamics simulation reveals the thermal stability, structural integrity and reversibility of BnV2, n = 6–10 at room temperature (300 K). The maximum practical hydrogen storage capacity at temperature up to 80 K and pressure ranges of 1–60 bar are found to store up to 8.75–10.78 wt% which is well above the target set by US-DOE (5.5 wt% by 2025). The results obtained from our investigations assure the potential of vanadium decorated small boron clusters for reversible hydrogen storage. 1. Introduction temperature of –40 to 80 ◦ C etc. [10–13]. In the recent past, nanostructured materials were studied extensively for solid-state hydrogen storage purposes in view of their fast reaction kinetics, stable thermodynamics and catalytic properties. Moreover, nanostructured materials could adsorb a considerable amount of hydrogen in molecular form due to their high surface to volume ratio and the weak dispersive interaction, which would result in easy desorption of adsorbed H2 molecules [14]. Extensive research work has been done on carbon-based nanomaterial such as carbon nanotube, fullerene, metal hydrides, metal–organic framework (MOFs), and co­ valent organic framework (COF) etc. for hydrogen storage purposes, but none of the said material could meet the US-DOE target [15–19]. More recently, boron-based clusters isovalent to carbonaceous compounds received considerable attention for hydrogen storage material due to their lightweight, catenation and suitable chemical and electronic properties. Recent studies suggested that the boron cluster decorated with suitable metal atoms could be a favourable storage medium due to its higher binding ability, which prevents the clustering of the metal atom over the host material [20,21]. Wu et al. showed that yttrium (Y) decorated B80 fullerene could adsorb up to 6.85 wt% of H2 through Dewar-Kubas interaction [22]. Dong et al. who theoretically predicted the hydrogen adsorption behaviour of titanium decorated B40 fullerene and found that the material showed excellent hydrogen binding capa­ bility up to 8.7 wt% with 0.2–0.4 eV/H2 adsorption energy [23]. During the last few decades, adequate use of fossil fuels for industrial and automotive applications has not only led to their depletion but also resulted in hazardous impacts on the global ecological system [1–3]. To deal with the above problem, hydrogen has emerged as one of the major alternative sources of energy because of its high abunduncy, high spe­ cific energy value and most importantly its carbon-free by-products. In addition, as compared to the traditional fossil fuel such as gasoline and diesel the efficiency of hydrogen is much more higher which further promotes hydrogen as a most promising candidate for the future energy need [4–8]. However, storage of hydrogen energy for automotive and industrial applications has been a major hurdle due to the challenging technology involved with it. Therefore, the major research focus in this area has been given to successfully develop storage media which show high gravimetric and volumetric density (5.5 wt% by 2025) and lenient chemical process under favourable thermodynamic conditions as pro­ posed by the US Department of Energy (US-DOE) [9]. Furthermore, there are some key parameters which should be maintained while choosing a potential solid-state hydrogen storage material viz. (i) large surface area of the host material under ambient conditions (ii) binding energy should be in the intermediate between physisorption and chemisorption mechanism (0.1–0.6 eV/H2) (iii) fast adsorption & desorption kinetics (1.5 kg H2/min) (iv) reversible operation * Corresponding author. E-mail address: [email protected] (S. Sahu). https://doi.org/10.1016/j.comptc.2022.113899 Received 14 June 2022; Received in revised form 9 September 2022; Accepted 26 September 2022 Available online 30 September 2022 2210-271X/© 2022 Elsevier B.V. All rights reserved. S.S. Ray et al. Computational and Theoretical Chemistry 1217 (2022) 113899 Scandium decorated B38 fullerene was studied by Liu et al., who reported that the B38 cluster could adsorb up to 7.57 wt% of H2 with average adsorption energy of 0.0224 eV [24]. Juan et al. reported that the cal­ cium decorated B36 showed potential hydrogen adsorption up to 4.97 wt % through an induced charge polarisation mechanism [25]. Moreover, other researchers have also widely reported numerous theoretical studies related to hydrogen adsorption in metal decorated boron clusters [25–29]. Besides, metal decorated small and medium-sized boron clusters (n < 20) have also shown better hydrogen storage capacity under ambient conditions than the larger boron clusters [30–33]. For instance, Ojha et al. predicted the hydrogen adsorption capacity of magnesium (Mg) decorated boron clusters and reported that there existed a closed shell type of interaction between Mg and H2 molecules, resulting in adsorp­ tion of 8.10 wt% of H2 molecules with an average adsorption energy range of 0.13–0.22 eV/H2 at ambient temperature and pressure [34]. Zhai et al. reported the hydrogen storage capacity of inverse sandwich like B6Ca2 and B8Ca2 clusters by performing Born-Oppenheimer mo­ lecular dynamics simulation and found that the these clusters could reversibly store and release maximum up to 14.2 wt% of H2 molecules with 0.13–0.15 eV/H2 adsorption energy at room temperature by satisfying the US DOE targets [35]. A similar kind of investigation was also done on the aromatic Ca2B8 complex by Du et al., and the results showed that 10.6 wt% of molecular hydrogen could be adsorbed on the complex (77 K), which could be efficiently released at ambient tem­ perature (300 K) as confirmed by ab initio molecular dynamics simu­ lations [36]. The hydrogen adsorption capacity of titanium (Ti) decorated B8 clusters was theoretically investigated by Liu et al., who reported up to 6 wt% of molecular hydrogen could be adsorbed on the complex with 0.24–0.35 eV/H2 of adsorption energy via Dewar-Kubas effects [37]. In our previous investigation, we reported the hydrogen adsorbing properties of scandium decorated small boron clusters employing molecular dynamics simulations, and we found that KubasNiu interaction attributed for adsorption of 9.43 wt% of H2 molecules with moderate average adsorption energy in the range 0.08–0.10 eV/H2 satisfying the US-DOE target for room temperature adsorption [38]. Wang et al. theoretically predicted the H2 adsorption in beryllium decorated small boron clusters, and they showed that these clusters could store up to 25 wt% of H2 via Dewar and van der Waals interactions with 0.10–0.50 eV/H2 adsorption energy [39]. However, hydrogen storage properties of small-sized boron clusters are scarcely reported, and there exist a considerable amount of small-sized boron clusters yet to be explored for hydrogen storage. Notwithstanding, experimental realization of hydrogen storage materials for commercial uses has been a challenging task for the researchers. Major issues such as poor thermo­ dynamics, metal clustering, desorption at extreme temperatures, reversibility and high-cost mechanisms are yet to be solved for vehicular or domestic use of hydrogen energy [1,40–41]. Previously, the electronic properties of the vanadium decorated small-sized boron clusters have been theoretically predicted. The structures are reported to be thermodynamically stable and possess bipyramidal geometry [42]. Because vanadium decorated clusters have been reported earlier to be potential candidates for hydrogen storage, therefore, in the present work we conducted a theoretical investigation on the performance of vanadium decorated small boron clusters (BnV2, n = 6–10) for hydrogen adsorption by employing dispersion corrected density functional theory [43,44]. Thermal stability, reversibility, and the desorption mechanism was examined by means of molecular dy­ namics simulation and van’t Hoff’s equation, respectively. used electronic structure calculation within the framework of density functional theory (DFT) to explore these properties. The detailed theory is discussed below. The conceptual density functional theory (C-DFT) was used to verify the thermodynamical stability and the reactivity of the optimized ge­ ometries upon calculating the global chemical reactivity descriptors (GCRDs) i. e hardness (η), and electrophilicity index (ω) [45–52]. Now, the chemical hardness (η) is computed by the ionization po­ tential (I) and electron affinity (A) using Koopmaan’s Theorem [53]. Now, the expression for chemical hardness is, η= I− A 2 (1) Similarly, the electrophilicity index can be expressed as. ω= χ2 (2) 2η χ= where I+A 2 Further, the kinetic stabilities of the studied geometries were confirmed by computing the energy gap (Eg) between their highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs). The average adsorption energy (Eads) and sequential desorption en­ ergy (Edes) for all hydrogen adsorbed structures can be defined by the following equations, 1 Eads = [{EHost + nEH2 } − EHost+nH 2 ] n Edes = 1 [{ 2EH2 + EHost+(n− 2 } 2)H2 − EHost+nH 2 (3) ] (4) Here EHost+nH2, EH2 and EHost are the total electronic energies of hy­ drogenated complex (BnV2-nH2), hydrogen molecule (H2) and host cluster (BnV2), respectively. The parameter n represents the total num­ ber of hydrogen molecules that are absorbed on the clusters. In addition, the term EHost+(n− 2)H2 represents the energy of the previous H2 molecule adsorbed, and n-2 represents two H2 molecules desorbed simultaneously. The gravimetric density is an important parameter which quantifies the capacity of a storage system. The hydrogen storage gravimetric density can be deduced using the following equation. H2 (wt%) = MH2 × 100 MH2 + MHost (5) Where MH2 indicates the mass of total number of adsorbed H2 mol­ ecules and MHost indicates the total mass of vanadium decorated boron clusters (BnV2, n = 6–10). The number of H2 molecules adsorbed on each vanadium atom of BnV2 cluster is determined by calculating the occupation number using the equation [54–56]. ] [ n(μ− Eads ) ∑Nmax kB T ngn e ] [ f = n=0 (6) n(μ− Eads ) ∑Nmax kB T n=0 gn e Where Nmax is the maximum binding number of H2 molecules to each V atom, n is the number of H2 molecules adsorbed, gn is the configura­ tional degeneracy for a given n, kB is the Boltzmann constant, Eads is the adsorption energy, and μ is the H2 gas-phase chemical potential at a given T and P obtained using the following equation [57]. ( ) P (7) μ = H 0 (T) − H 0 (0) − TS0 (T) + kB Tln P0 2. Computational details To investigate the characteristics of a potential hydrogen storage system, essential properties such as stability, energy and storage ca­ pacity of the host cluster are, in general, calculated along with addi­ tional thermodynamical properties to justify its practical usability. We Here H0, S0 are the enthalpy and entropy of H2 at pressure P0 = 1 bar obtained from Reference [58]. 2 S.S. Ray et al. Computational and Theoretical Chemistry 1217 (2022) 113899 Table 1 Average bond lengths between Boron-Boron (dB-B), Boron-Vanadium (dB-V), Vanadium-Vanadium (dV-V), Vanadium-Hydrogen (dV-H), Hydrogen-Hydrogen (dH-H) in Å. Cluster dB-B (Å) dB-V (Å) dV-V (Å) B6Ti2 B6V2-10H2 B7V2 B7V2-10H2 B8V2 B8V2-10H2 B9V2 B9V2-10H2 B10V2 B10V2-10H2 1.579 1.591 1.547 1.556 1.533 1.533 1.587 1.574 1.604 1.612 2.099 2.112 2.208 2.244 2.309 2.381 2.260 2.284 2.432 2.554 2.768 2.782 2.605 2.703 2.298 2.579 2.560 2.706 2.211 2.634 dV-H (Å) dH-H (Å) 2.853 0.77 2.731 0.78 2.851 0.78 2.871 0.78 2.970 0.78 [65]. Bader’s Quantum Theory of Atoms in Molecules (QTAIM) was used to analyse the nature of the interaction between the hydrogen molecules and sorption centres [66]. Thermodynamics reversibility of adsorbed hydrogen molecules on the vanadium decorated boron clusters (BnV2, n = 6–10) were deter­ mined by performing molecular dynamics simulation using Atomcentered Density Matrix Propagation (ADMP). The time steps for the simulation are set to one femtosecond (△t = 1 fs), and the step size has been specified to a maximum of 1000 steps for each trajectory. The temperature is maintained for thermostatic simulations by applying the velocity scaling method during the simulation [67–69]. We used Gaussian 09 program suit to perform all the DFT calculations [70]. Fig. 1. Optimized geometry of bare and hydrogenated B6V2 cluster (a) B6V2, (b) B6V2-2H2, (c) B6V2-4H2,(d) B6V2-6H2, (e) B6V2-8H2, and (e) B6V2-10H2. 3. Results & discussion 3.1. Geometry & stability Generally, the hydrogen adsorption mechanism is governed by noncovalent weak interactions such as van der Waals forces. Therefore to get a stable geometry and reliable adsorption energy, all the geometries and frequency calculations were carried out using exchange correla­ tional functional Perdew–Burke-Ernzerhof (PBE) based on generalised gradient approximation (GGA) and adding Grimme’s dispersion correction method to density functional theory (DFT-D3) [59–60]. PBED3, which includes empirical dispersion and long-range corrections, has been proven as a reliable method to study the weak non-covalent interaction [61–63]. So the bare and hydrogen adsorbed structures were optimized using the PBE-D3 method along with the standard splitvalence basis set with diffuse and polarization function 6–311++G(d,p) [64]. The nature of the bonding between the adsorbed hydrogen and the metal atom are explored by computing the NBO charges on each atom In order to examine the hydrogen adsorption and storage capacity of vanadium decorated small boron clusters (BnV2, n-6–10), we reoptimize the host clusters with reference to the previous report using the density functional theory (DFT) in conjunction with Grimme’s dispersion correction and PBE-D3/6–311++G(d,p) basis set to. The results of optimized geometries are found in good agreement with previous report [42]. Then hydrogen molecules are sequentially added to host clusters, and the complexes are re-optimized at the same level of theory. The stable geometries of hydrogenated B6V2 clusters and BnV2-10H2, n = 7–10 are illustrated in Figs. 1 and 2 respectively, and the other optimized hydrogenated complexes are presented in the Figure S1 (Supplementary Information). The calculated geometrical parameters Fig. 2. Optimized geometry of hydrogenated BnV2 cluster (a) B6V2,-10H2 (b) B7V2-10H2, (c) B8V2-10H2,(d) B9V2-10H2, and (e) B10V2-10H2. 3 S.S. Ray et al. Computational and Theoretical Chemistry 1217 (2022) 113899 length is found in the range of 2.58–2.78 Å which is larger than the ionic radius of V which inhibits any clustering over the complexes. The H2 molecules are found to be adsorbed on the sorption site (V) in the quasimolecular form at an average distance in the range of 2.731 Å-2.970 Å which is recommended by US-DOE for molecular adsorption. It is evident form the Table 1 the structures of the host clusters remain almost undistorted throughout the adsorption process indicating their chemical stabilities. Stability and the reactivity of the clusters can also be explained in terms of conceptual DFT based global chemical reactivity descriptors (GCRDs) i. e hardness (η) and electrophilicity index (ω). The GCRD pa­ rameters are computed using Equations (1) and (2) and are recorded in Table 2. For the studied clusters, the hardness values are found to in­ crease while the electrophilicity values decrease upon gradual addition of H2 molecules. For example, in B6V2 cluster the hardness increases by 15 % upon addition of 10H2 molecules while the value of ω decreases almost by 11 %. Similarly, hardness of B10V2 increases by 57 % upon hydrogenation whereas its electrophilicity decreases by 66 %. This im­ plies the chemical stability of the studied clusters following the principle of maximum hardness (MHP) and minimum electrophilicity principle Table 2 Calculated hardness (η), electrophilicity index (ω), and HOMO-LUMO energy gap (Eg) of vanadium doped boron clusters (BnV2, n = 6–10) as well as hydro­ genated clusters. Cluster η (eV) ω (eV) Eg (eV) B6V2 B6V2-10H2 B7V2 B7V2-10H2 B8V2 B8V2-10H2 B9V2 B9V2-10H2 B10V2 B10V2-10H2 0.65 0.77 0.56 0.56 0.39 0.37 0.39 0.41 0.17 0.40 10.58 9.39 16.70 18.17 31.54 30.21 27.25 25.69 76.40 25.53 1.31 1.55 1.12 1.12 0.78 0.74 0.79 0.83 0.34 0.81 are shown in Table 1. It is found that maximum of five numbers H2 molecules were adsorbed on each vanadium atom with H–H bond length in the range of 0.75–0.85 Å, which is slightly elongated as compared to that of the bare H2 molecule. In addition, the V-V bond Fig. 3. Average adsorption energy and sequential desorption energies of hydrogenated BnV2, n = 6–10 clusters. Fig. 4. Partial density of states on V and H atoms of B6V2-nH2 (n-2,10), (a) B6V2-2H2 and (b) B6V2-10H2. 4 S.S. Ray et al. Computational and Theoretical Chemistry 1217 (2022) 113899 energy per H2 molecule is found to reduce approximately to 0.04 eV with a maximum number of H2 adsorption, and with the release of the H2 molecules, the geometries of the host clusters almost remain intact, which implies the reversibility of the studied system. Table 3 Average NBO charges on each atom of BnV2 (n = 6–10) clusters before and after H2 adsorption. Cluster B6V2 B7V2 B8V2 B9V2 B10V2 Before H2 adsorption After H2 adsorption Charge on B Charge on V Charge on B Charge on V 0.10 − 0.06 0.19 0.15 0.11 0.16 0.03 0.06 0.01 0.05 − − − − − − − − − − 0.03 0.05 0.04 0.03 0.02 1.19 0.83 1.12 0.77 1.21 Charge on H 3.3. Partial density of state (PDOS) analysis 0.06 0.04 0.08 0.04 0.09 Partial density of state (PDOS) plots for vanadium and hydrogen atoms of hydrogenated B6V2, clusters with firsts and last hydrogen molecules adsorbed on each vanadium atom are presented in the Fig. 4 and PDOS of other hydrogenated clusters are provided in Figure S3-S6 (Supplementary Information). From PDOS plot it is observed that upon adsorption of the 1st H2 molecule on each vanadium atom of B6V2, the σ orbital of the hydrogen molecule overlap with the 3d orbital of V atom at − 12.6 eV below the Fermi level and the σ * orbital of H2 interacts with orbital of V above the Fermi level (Fig. 4(a)) indicating Kubas type of interaction between hydrogen and V atom. According to the Kubas mechanism, the occupied orbital of the H2 interacts with the vacant 3d orbital of the V atom and a small charge transfer occurs from H2 mole­ cule with back donation of charge from partially filled 3d orbitals of V atom to the unfilled σ * orbital of H2 molecules [74,75]. However, with increase in the hydrogen content over each vanadium atom (5H2) the intensity of the σ orbital increases and move closer to the Fermi level (Fig. 4b) and splits in to number of intense peaks near − 13.3 eV to − 9 eV implying the interaction getting weaker. This weak inter­ action can be explained through Niu-Rao-Jena mechanism in which induced dipole develops in the H2 molecule due to the charge polar­ isation [76,77]. Similar kinds of observation were found for all other studied clusters. (MEP) [71,72]. In addition to that, the computed HOMO-LUMO energy gap (Eg) also shows a gradual increase upon addition of the H2 molecules (Figure. S2) during the adsorption process also indicating the molecular stability of the complexes. 3.2. Adsorption energy The energetic of the adsorption process in the studied clusters is discussed through the average hydrogen adsorption energy and suc­ cessive desorption energies calculated using Equations (3) and (4), respectively. We add H2 molecules sequentially over the sorption sites of BnV2 n = 6–10 clusters and find that 10 H2 molecules get adsorbed on both V atoms of the clusters with an average adsorption energy range of 0.17–0.26 eV/H2, which implies the adsorption process to be phys­ isorptive in nature. The variation of Eads with the number of adsorbed H2 molecules is depicted in Fig. 3. It can be noted that as the number of H2 molecules increases, the value of Eads follows a decreasing trend. For instance, when two H2 molecules are adsorbed on the B6V2 clusters, Eads value is computed to be 0.33 eV/H2. When the number of H2 molecules gradually increases, the Eads value reduces to 0.17 eV/H2 with an increasing V-H2 distance due to steric hindrance [73]. For practical use of hydrogen storage, delivery of H2 gas necessarily depends on the efficient desorption of hydrogen molecules. The suc­ cessive desorption energy (Edes) (Fig. 3) is also found to show the same pattern with the number of H2 molecules as Eads dose. The desorption 3.4. Interactions & bonding mechanism We calculated the average natural bond orbital (NBO) charges before and after adsorption to understand the bonding mechanism in the studied complexes. The average NBO charges on B, V and H atoms are listed in Table 3. During the adsorption process, about 0.05–0.20 eu of positive NBO charges are transferred from the V atom to the B atom resulting in an electric field around the former. This electric field Fig. 5. Electrostatic potential (ESP) plots of bare and hydrogen adsorbed B6V2 cluster. (a) B6V2, (b) B6V2-2H2, (c) B6V2-4H2, (d) B6V2-6H2,(e) B6V2-8H2, and (f) B6V2-10H2. 5 S.S. Ray et al. Computational and Theoretical Chemistry 1217 (2022) 113899 induces polarization in H2 molecules, causing them to bind to the va­ nadium atom via charge polarization mechanism as proposed by NiuRao-Jena [76,77]. The fact is also supported by the electrostatic po­ tential maps (ESP) analysis discussed below. The ESP plots for B6V2 and BnV2, n-7–10, and their hydrogen adsorbed derivatives, are presented in Fig. 5 and Figure S7 (Supple­ mentary Information), respectively. In the ESP map, the red colour at­ tributes to the accumulation of electron density while the blue colour Table 4 Calculated electron density in (ρ) a.u.and ∇2 ρ in a.u at BCP of (B,V) and (V, H). Cluster ρV− B6V2-10H2 B7V2-10H2 B8V2-10H2 B9V2-10H2 B10V2-10H2 0.0884 0.0719 0.0584 0.0756 0.0718 B ∇2 ρV− B 0.1206 0.1050 0.0975 0.0893 0.0923 ρV− H 0.0364 0.0483 0.0618 0.0570 0.0601 ∇2 ρV− H 0.1433 0.2030 0.2497 0.2276 0.2449 Fig. 6. Potential energy trajectories BnV2-10H2, n = 6–10 clusters at 0 K, 77 K, 300 K and TD[max] temperatures respectively. 6 S.S. Ray et al. Computational and Theoretical Chemistry 1217 (2022) 113899 attributes to electron density depletion. From Fig. 4, it can be noted that the region over each vanadium atom is marked by dark blue colour, signifying the electron depletion region as compared to the boron cluster as pointed out from the NBO analysis. On adsorption of the first and second H2 molecules, the colour changes from dark blue to light blue. This comes about due to the electron density variation at the adsorption sites leading to the Kubas-type bonding. Upon further adding H2 mole­ cules to the system, we do not observe any significant colour variation except the ESP of H2 changes from light-blue to bluish-green, which signifies that these H2 molecules are physisorbed due to the charge polarization mechanism (Niu-Rao-Jena interaction) [76,77]. A similar observation is also found for all the other studied clusters. Further, the nature of interaction between the sorption centre (V atom) and the adsorbed H2 molecules is characterized by analyzing the topological parameters from the outcomes of Bader’s quantum theory of atom in molecules calculation (QTAIM) [66]. The parameters such as electron density (ρ) and Laplacian of electron density (∇2ρ) at the bond critical point (BCP) are computed and presented in Table 4. The calcu­ lated value of ρ < 0.20 with a positive value of ∇2ρ at BCP of V-H2 bespeaks a closed shell type (weak non-covalent) interaction between the metal atom (V) and adsorbed H2 molecule [78–80]. This noncovalent interaction is mainly due to the charge polarization mecha­ nism as confirmed from the NBO charge distribution and ESP analysis. During the interaction, the interatomic distance of adsorbed H2 mole­ cules is found to be slightly elongated (up to 0.78 Å). However, the average values of ρ over the H–H in all the hydrogenated clusters are found to be almost same as that over the H–H in the isolated hydrogen molecules which implies that the adsorbed hydrogens are in molecular form (Table S1). The values of ρ and ∇2ρ are found in the range of 0.058–0.088 a.u and 0.089–0.120 a.u, respectively, suggesting all the studied clusters can bind the H2 molecules via non-covalent interactions. Table 5 Thermodynamically usable hydrogen capacity. NTheory is the number of adsor­ bed H2 molecules in calculation. Nads and Ndes the number of H2 molecules adsorbed at (100 K − 60 bar) and desorbed (300 K − 1 bar) respectively. Nuse (=Nads - Ndes) represents the usable number of H2 molecules. GTheory and G100k− 60bar represents the theoretical and practical hydrogen wt% at adsorption conditions respectively. Cluster NTheory Nads Ndes Nuse GTheory G100K-60bar B6V2-10H2 B7V2-10H2 B8V2-10H2 B9V2-10H2 B10V2-10H2 10 10 10 10 10 9.07 9.62 6.01 7.92 7.56 0.05 0.83 0.51 1.64 2.59 9.02 8.79 5.50 6.28 4.97 10.78 10.19 9.66 9.19 8.75 9.88 9.84 6.04 7.42 6.76 Equation (6) by employing the empirical value of the chemical potential of H2 gas. We chose 100 K/60 bar for adsorption and 400 K/3 bar for the desorption condition for all the clusters to estimate the usable number of H2 molecules, and the values are presented in Table 5. The variations of H2 occupation number with a finite range of temperatures and pressures are shown in Fig. 7. It is observed that the adsorption occurs at high pressure, whereas the desorption occurs at a lower pressure as the chemical potential of the H2 gas increases with pressure. Therefore, the H2 molecules are released at a constant temperature with lower pressure [46]. It is found that, at a temperature of 80 K and a pressure range of 1–60 bar, all the studied clusters possess 10H2 molecules leading to a maximum H2 storage capacity of 8.75 wt% − 10.78 wt%, which are well above the target set by US-DOE (5.5 wt% by 2025). As temperature rises beyond 80 K, the H2 molecules start desorbing from the host clusters, and at 400 K in the pressure range of 1–3 bar, almost all the hydrogen molecules desorbed from the host cluster. At the storage condition (100 K and 60 bar) all the studied cluster shows a gravimetric storage ca­ pacity up 6.76–9.88 wt%. Moreover, we find that the gravimetric stor­ age capacity of all the studied clusters at a temperature and pressure range of 120 K-160 K and 30–60 bar are closed to the target set by USDOE. The computed average adsorption energy, average desorption tem­ perature and hydrogen gravimetric density for the studied clusters can be found suitable for a practical hydrogen storage system. We have also compared these parameters with the previously reported work on similar system and the data are presented in the Table 6. Practical desorption temperature (TD) for vanadium decorated boron clusters is estimated using the van’t Hoffs equation (Equation (8)) for the range of pressure (1–5 atm) with an increment of 0.5. )− 1 ( )( Eads ΔS − lnP TD = (8) R kB 3.5. Molecular dynamics simulations We examine the thermal stability and reversibility of the studied clusters at different thermodynamic conditions for feasible hydrogen storage using ADMP-molecular dynamics (MD) simulations. The simu­ lation is carried out at four different temperatures i.e 0 K, 77 K, 300 K and maximum desorption temperature (TD[max]), for 1 ps time scale employing the velocity scaling method. The variation of potential en­ ergy with time is depicted in Fig. 6 and the snapshots of BnV2-10 H2, n = 6–10 systems at different temperatures are presented in Figure S8-S12 (Supplementary Information). The MD simulations reveal that at low temperatures (0 & 77 K), the system can hold almost all the adsorbed H2 molecules. For example, at 77 K, nearly-two H2 molecules move away from the sorption centres while all others are retained on the surface, resulting in hydrogen uptake capacity of up to 10 wt%. On the other hand, while we increase the temperature to 300 K, the hydrogen molecules start desorbing from the host clusters, and at the end of the simulation, a maximum number of H2 molecules gets desorbed from the host clusters without distorting the parent clusters. For instance, at 300 K, only three H2 molecules remain adsorbed to the B6V2 clusters. To examine the thermal stability of the host clusters, we analyse the variation of bond distance between the two atoms of the host clusters. In Figure S13 (Supplementary Information), we depict variations 〈dB − B〉, 〈dB− V〉, and 〈dV− V〉 bond distances at 300 K temperature. It is observed that the host clusters remain stable with minimal fluctuation in all the bond distances, which assures the thermal stability and reversibility of the host clusters during the desorption process. Where Eads is the calculated H2 adsorption energy, kB is the Boltz­ mann Constant, △S is the change in hydrogen entropy from gas to the liquid phase. R is the gas constant, and P is the pressure (1 atm). The variation of desorption temperature with equilibrium pressures is presented in Fig. 8 and Figure S14 (Supplementary Information), respectively. It is observed that the average value of TD fall in a range of 319.8 K-582.1 K under standard atmospheric pressure. For all the studied clusters, the TD[avg] is found to be higher than the room tem­ perature, which implies that H2 molecules do not dissociate at small thermal fluctuations. It can also be noted that the calculated values of desorption temperature follow an increasing trend with increasing pressure. The molecular dynamics simulations also support the computed results for the practical desorption temperature. 4. Conclusion 3.6. Thermodynamically usable hydrogen storage This study theoretically predicted the reversible hydrogen storage capacity of vanadium decorated small boron clusters (BnV2, n = 6–10), employing dispersion corrected density functional study and molecular To determine the practical usable hydrogen capacities at ambient thermodynamic conditions, the occupation number (f) is calculated at various ranges of temperature and pressure (40–400 K & 1–60 bar) using 7 S.S. Ray et al. Computational and Theoretical Chemistry 1217 (2022) 113899 Fig. 7. Occupation number of H2 molecules as a function of the pressure and temperature on hydrogenated BnV2, n = 6–10 clusters. Table 6 Hydrogen storage par ameters comparison for various nanomaterials. System Total number of adsorbed hydrogen molecule Average adsorption energy per H2 (eV) Average desorption temperature (K) Gravimetric density(%) TiBn Clusters [28] Mg2Bn, n = 4–14 [34] B8Ti Cluster [37] BnSc2, n-3–10 [38] Mx–B6H6 Complexes (M = Y − Mo, Ru − Ag, x = 1–2 [81] Y + B40 [82] Sc2–C6H6 [83] Experimental Graphene based Nano composite [84] CNT + Pd [85] Present work 4 0.31 700 K 6.12 5 0.19 300 K 8.10 6 8 0.24 0.10 300 K 300 K 6.22 9.43 12 0.25 >300 K 8.86 5 8 – 0.21 0.35 – 281 300 K – 5.8 8.76 >5 10 0.26 300 K Fig. 8. Variation of desorption temperature with equilibrium pressure for B6V210H2 clusters. The black, red and blue lines represent minimum, average and maximum temperatures respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.) 6 10.78 Topological analysis revealed the interaction between the host clusters and vanadium atoms to be weak non-covalent. ADMP-MD simulations suggested that clusters could adsorb a considerable amount of H2 at low temperature (77 K), giving rise to storage capacity up to 10 wt% which was well above the target of US-DOE. At 300 K, most of the hydrogen molecules got desorbed without distorting the host clusters, implying their thermal stability and reversibility. The H2 occupation number dynamics simulations. The stabilities of the hydrogenated clusters were confirmed by their enhanced chemical hardness and HOMO-LUMO gaps. The H2 molecules were found to adsorb on BnV2, n = 6–10 via Kubas-Niu interaction with an average adsorption energy range of 0.17–0.26 eV/H2 inferring the adsorption process to be physisortive and quasi-molecular. The fact was supported by NBO and ESP analyses. 8 S.S. Ray et al. Computational and Theoretical Chemistry 1217 (2022) 113899 calculation indicated that, at a temperature of 80 K and a pressure range of 1–60 bar, all the studied clusters possessed 10H2 molecules leading to a maximum H2 storage capacity of 8.75 wt% − 10.78 wt%, and at a temperature and pressure range of 120 K-160 K and 30–60 bar the storage capacities were close to 5.5 wt%. The average desorption tem­ perature (TD ) of all the studied clusters were found in a range of 319.8 K582.1 K under standard atmospheric pressure. Based on our result, we can predict that BnV2, n = 6–10 clusters can be considered as promising candidates for the reversible hydrogen storage medium under ambient thermodynamic conditions. [12] R. Rahimi, M. Solimannejad, High-Performance hydrogen storage properties of Lidecorated B2N2 nanosheets: a periodic density functional theory study, Energy Fuels 35 (8) (2021) 6858–6867. [13] Y. Zhang, P. Liu, X. Zhu, Z.e. Liu, A reversible hydrogen storage material of Lidecorated two-dimensional (2D) C4N monolayer: first principles calculations, Int. J. Hydrogen Energy 46 (65) (2021) 32936–32948. [14] A. Bhattacharya, S. Bhattacharya, Exploring N-rich phases in LixNy clusters for hydrogen storage at nanoscale, J. Phy. Chem. Lett. 6 (18) (2015) 3726–3730. [15] M. Mohan, V.K. Sharma, E.A. Kumar, V. Gayathri, Hydrogen storage in carbon materials—a review, Energy Storage 1 (2) (2019) e35. [16] J. Guo, Z. Liu, S. Liu, X. Zhao, K. Huang, High-capacity hydrogen storage medium: Ti doped fullerene, Appl. Phys. Lett. 98 (2) (2011) 023107. [17] H.M. Cheng, Q.H. Yang, C. Liu, Hydrogen storage in carbon nanotubes, Carbon 39 (10) (2001) 1447–1454. [18] L.J. Murray, M. Dincă, J.R. Long, Hydrogen storage in metal–organic frameworks, Chem. Soc. Rev. 38 (5) (2009) 1294–1314. [19] E. Klontzas, E. Tylianakis, G.E. Froudakis, Hydrogen storage in 3D covalent organic frameworks. a multiscale theoretical investigation, J. Phys. Chem. C. 112 (24) (2008 Jun 19) 9095–9098. [20] E. Fakioglu, Y. Yurum, T.N. Veziroglu, A review of hydrogen storage systems based on boron and its compounds, Int. J. Hydrogen Energy 29 (2004) 1371–1376. [21] J. Joseph, V.S. Sivasankarapillai, S. Nikazar, M.S. Shanawaz, A. Rahdar, H. Lin, G. Z. Kyzas, Borophene and boron fullerene materials in hydrogen storage: opportunities and challenges, ChemSusChem. 13 (15) (2020) 3754–3765. [22] W.W. Wu, Z.Y. Tian, S.L. Dong, Yttrium-dispersed B80 fullerenes: Promising materials for hydrogen storage, EPL 109 (5) (2015) 56004. [23] H. Dong, T. Hou, S.T. Lee, Y. Li, New Ti-decorated B40 fullerene as a promising hydrogen storage material, Sci. Rep. 5 (2015) 09952. [24] P. Liu, F. Liu, Q. Wang, Q. Ma, DFT simulation on hydrogen storage property over Sc decorated B38 fullerene, Int. J. Hydrogen Energy 43 (42) (2018) 19540–19546. [25] Liu P, liu F, Peng Y, Wang Q, Juan R. A DFT study of hydrogen adsorption on Ca decorated hexagonal B36 with van der Waals corrections. Physica E: Lowdimensional Systems and Nanostructures 2019;114:113576. [26] L. Si, C. Tang, The reversible hydrogen storage abilities of metal Na (Li, K, Ca, Mg, Sc, Ti, Y) decorated all-boron cage B28, Int. J. Hydrogen Energy 42 (26) (2017) 16611–16619. [27] N. Song, J. Lv, Y. Wang, B24 cluster as promising material for lithium storage and hydrogen storage applications, Comput. Mater. Sci. 77 (2013) 31–34. [28] P.L. Rodríguez-Kessler, A.R. Rodríguez-Domínguez, D. MacLeod-Carey, A. MuñozCastro, Exploring the size-dependent hydrogen storage property on Ti-Doped Bn Clusters by diatomic deposition: temperature controlled H2 Release, Adv. Thoe Simul 4 (7) (2021) 2100043. [29] T.B. Tai, M.T. Nguyen, A three-dimensional aromatic B6Li8 complex as a high capacity hydrogen storage material, Chem. Commun. 49 (9) (2013) 913–915. [30] H. Huang, B. Wu, Q. Gao, P. Li, X. Yang, Structural, electronic and spectral properties referring to hydrogen storage capacity in binary alloy ScBn (n=1-12) clusters, Int. J. Hydrogen Energy 42 (2017) 21086–21095. [31] S. Banerjee, G. Periyasamy, S.K. Pati, Density functional theoretical investigation on structure, optical response and hydrogen adsorption properties of B9/metal B9 clusters, Phys. Chem. Chem. Phys. 15 (2013) 8303–8310. [32] Hydrogen capability of bimetallic boron cycles:A DFT and ab initio MD study. International Journal of Hydrogen Energy 2019;44:6763-6772. [33] J. Du, X. Sun, G. Jiang, Hydrogen storage capability of cage like Li3B12 clusters, J. Appl. Phys. 127 (5) (2020) 054301. [34] A. Kumar, N. Vyas, A.K. Ojha, Hydrogen storage in magnesium decorated boron clusters (Mg2Bn, n=1-14): a density functional theory study, Int. J. Hydrogen Energy 46 (2020) 19023–19030. [35] Y.-J. Wang, G.-L. Wang, M.-M. Guo, C.-Q. Miao, H.-P. Chen, H.-J. Zhai, The highcapacity hydrogen storage of B6Ca2 and B8Ca2 inverse sandwiches, Int. J. Hydrogen Energy 46 (47) (2021) 24225–24232. [36] J. Du, G. Jiang, An aromatic Ca2B8 complex for reversible hydrogen storage, Int. J. Hydrogen Energy 46 (36) (2021) 19023–19030. [37] P. Liu, Y. Zhang, X. Xu, F. Liu, J. Li, Ti decorated B8 as a potential hydrogen storage material: a DFT study with van der Waals corrections, Chem. Phys. Lett. 765 (2021) 138277. [38] S.S. Ray, S.R. Sahoo, S. Sahu, Hydrogen storage in scandium doped small boron clusters (BnSc2, n=3-10): a density functional study, Int. J. Hydrogen Energy 46 (2019) 19023–19030. [39] Y.-J. Wang, L.i. Xu, L.-H. Qiao, J. Ren, X.-R. Hou, C.-Q. Miao, Ultra-high capacity hydrogen storage of B6Be2 and B8Be2 clusters, Int. J. Hydrogen Energy 45 (23) (2020) 12932–12939. [40] Q. Sun, Q. Wang, P. Jena, Y. Kawazoe, Clustering of Ti on a C60 surface and its effect on hydrogen storage, J. Am. Chem. Soc. 127 (42) (2005) 14582–14583. [41] H.T. Nai, P.K. Jha, B. Chakraborty, Int. J. Hydrogen Energy (2022), https://doi. org/10.1016/j.ijhydene.2022.08.084. [42] L.N. Zhang, J. Jia, H.S. Wu, Structural and electronic properties of V2Bn (n = 1–10) clusters, Chem. Phys. 459 (2015) 131–136. [43] C. Guo, C. Wang, Computational investigation of hydrogen storage on B5V3, Mol. Phys. 116 (10) (2018) 1290–1296. [44] S. Nachimuthu, L. He, H.J. Cheng, R.D. Tiono, J.C. Jiang, A first-principles study on double-sided decorated boron–nitrogen co-doped graphene by vanadium for enhanced low-temperature reversible hydrogen storage, Sustainable Energy Fuels 5 (7) (2021) 2159–12156. [45] P.K. Chatraj, Chemical reactivity theory: a density functional view, Taylor and Francis.CRC Press, Boca Raton, FL, 2009. CRediT authorship contribution statement Shakti S Ray: Conceptualization, Methodology, Software, Valida­ tion, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing. Rakesh K Sahoo: Conceptualization, Investigation, Data curation, Validation. Sridhar Sahu: Conceptualiza­ tion, Resources, Formal analysis, Validation, Supervision, Project administration. Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Data availability Data will be made available on request. Acknowledgement We acknowledge the financial support from Science & Engineering Research Board (SERB), DST, India under grant no. EMR/2014/000141. The Technical Education Quality Improvement Programme-III, Gov­ ernment of India is also acknowledged for the partial financial support. The authors also acknowledge the Indian Institute of Technology (Indian School of Mines), Dhanbad for providing support and other research facilities. Appendix A. Supplementary material Supplementary data to this article can be found online at https://doi. org/10.1016/j.comptc.2022.113899. References [1] P. Jena, Materials for hydrogen storage: past, present, and future, J. Phys. Chem. Lett. 2 (3) (2011) 206–211. [2] Upadhyay D, Roondhe B, Pratap A and Jha PK. Two-dimensional delafossite cobalt oxyhydroxide as a toxic gas sensor. Applied Surface Science 2019;476:198-204. [3] S. Patel, P. Patel, D. Chodvadiya, N.N. Som, P.K. Jha, J. Mol. Liquids 352 (2022) 11872. [4] E. David, An overview of advanced material for hydrogen storage, J. Mat. Proc. Technol. 163 (2005) 16977. [5] I. Sreedhar, M.K. Kamani, M.B. Kamani, M.B. Reddy, A. Venugopal, A bird’s eye view on proces and engineering aspects of hydrogen storage, Renew. Sustain. Energy Rev. 91 (2018) 838–860. [6] L. Schlapbach, A. Züttel, Hydrogen-storage materials for mobile applications, Nature 414 (6861) (2001) 353–358. [7] A. Züttel, Materials for hydrogen storage, Mater. hydrogen storage. Mater. Today 6 (9) (2003) 24–33. [8] Hircher M. Handbook of hydrogen storage: New materials for future energy storage. Winheim: Wiley-VCH Verlang GmbH & Co. KGa; 2010. [9] https://www.energy.gov/eere/fuelcells/hydrogen-storage. [10] R.K. Sahoo, S. Sahu, Reversible hydrogen storage capacity of Li and Sc doped novel C8N8 cage: Insights from density functional theory, Int. J. Energy Res. 1–16 (2022), https://doi.org/10.1002/er.8562. [11] M.R. Mananghaya, Hydrogen saturation limit of Ti-doped BN nanotube with B-N defects: an insight from DFT calculations, Int. J. Hydrogen Energy 43 (22) (2018) 10368–10375. 9 S.S. Ray et al. Computational and Theoretical Chemistry 1217 (2022) 113899 [46] R.G. Parr, Absolute hardness: companion parameter to absolute electronegativity, J. Am. Chem. Soc. 105 (1983) 7512–7756. [47] Sen KD, Jorqenson CK. Electronegativity; Structure and Bonding. 66;Springer: Berlin,1987. [48] R.G. Parr, L.V. Szentpaly, S. Liu, Electrophilicity Index, J. Am. Chem. Soc. 121 (1999) 1922–1924. [49] S. Vadlakar, D. Chodvadiya, N.N. Som, N.K. Vyas, P.K. Jha, B. Chakraborty, An Abinitio Study of the C18 nanocluster for Hazardous Gas Sensor application, Chem. Select 7 (2022). [50] M.A. Hossain, M.R. Hossain, M.K. Hossain, J.I. Khandaker, F. Ahmed, T. Ferdous, M.A. Hossain, An ab initio study of the B35 boron nanocluster for application as atmospheric gas (NO, NO2, N2O, NH3) sensor, Chem Phys Lett 754 (2020), 137701. [51] Y. Liu, C. Liu, A. Kumar, A selective NO sensor based on the semiconducting BC2N nanotubes: a computational study, Mol. Phys. 118 (2020) e1798528. [52] R.K. Sahoo, P. Kour, S. Sahu, Reversible hydrogen storage capacity of Sc and Y functionalized [1, 1] paracyclophane: insights from density functional study, Int. J. Hydrogen Energy 47 (2022) 29881–29895. [53] T.A. Koopmans, U ¨ber die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms, Physica. 1 (1934) 104–113. [54] H. Lee, M.C. Nguyen, J. Ihm, Titanium-functional group complexes for highcapacity hydrogen storage materials, Solid State Commun. 146 (2008) 431–434. [55] H. Lee, W.I. Choi, M.C. Nguyen, M.H. Cha, E. Moon, J. Ihm, Ab initio study of dihydrogen binding in metal-decorated polyacetylene for hydrogen storage, Phys. Review B. 76 (2007), 195110. [56] S. Kumar, T.J. Dhilip Kumar, Electronic structure calculations of hydrogen storage in lithium-decorated metal–graphyne framework, ACS Appl. Mater. Interfaces 9 (2017) 28659–28666. [57] T. Wassmann, A.P. Seitsonen, A.M. Saitta, M. Lazzeri, F. Mauri, Structure, stability, edge states, and aromaticity of graphene ribbons, Phys. Rev. Lett. 101 (9) (2008), 096402. [58] Lide D. R. CRC handbook of chemistry and physics (Vol. 85). CRC press (Ed.) 2004. [59] S. Grimme, J. Antony, S. Ehrlich, H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu, J. Chem. Phys. 132 (2010), 154104. [60] S. Grimme, J. Mollemann, DFT-D3 Study of Some Molecular Crystals, J. Phys. Chem. C 118 (2014) 7615–7621. [61] Lee K , Berland K , M. Yoon M, Andersson S, Schroder E , P. Hyldgaard and Lundqvist BI. Benchmarking van der Waals density functionals with experimental data: potential-energy curves for H2 molecules on Cu(111), (100) and (110) surfaces. J Phys Condens Matter 2012;24:424213. [62] M. Kocman, P. Jurecka, M. Dubecky, M. Otyepka, Y. Chobc, K.S. Kim, Choosing a density functional for modeling adsorptive hydrogen storage: reference quantum mechanical calculations and a comparison of dispersion-corrected density functionals Phys, Chem. Chem. Phys. 17 (2015) 6423–6432. [63] F, Bakhshi, N, Farhadian Co-doped graphene sheets as a novel adsorbent for hydrogen storage: DFT and DFT-D3 correction dispersion study Int J Hydrogen Energy 2018;43:8355-64. [64] R. Konda, V. Kalamse, A. Deshmukh, A. Chaudhari, Closoborate-transition metal complexes for hydrogen storage, RSC Adv. 5 (2015) 99207–99216. [65] Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Landis CR and Weinhold F, NBO 6.0. 2013. Theoretical Chemistry Institute, University of Wisconsin, Madison, WI, USA. [66] Bader RFW. Atoms in Molecules.A Quantum Theory 1990; Clarendon press. Oxford. [67] H.B. Schlegelm, J.M. Millam, S.S. Iyengar, G.A. Voth, A.D. Daniels, G.E. Scuseria, M.J. Frisch, Ab initio molecular dynamics: propagating the density matrix with Gaussian orbitalsJ, Chem. Phys. 114 (2001) 9758–9763. [68] S.S. Iyengar, H.B. Schlegel, J.M. Millam, G.A. Voth, G.E. Scuseria, M.J. Frisch, Ab initio molecular dynamics: Propagating the density matrix with Gaussian orbitals. II. Generalizations based on mass-weighting, idempotency, energy conservation and choice of initial conditions, The J. Chem. Phy. 115 (22) (2001) 10291–10302. [69] H.B. Schlegel, S.S. Iyengar, X. Li, J.M. Millam, G.A. Voth, G.E. Scuseria, M.J. Frisch, Ab initio molecular dynamics: Propagating the density matrix with Gaussian orbitals III. Comparison with Born-Oppenheimer dynamics, J. Chem. Phy. 117 (19) (2002) 8694–8704. [70] Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, CheesemanJR et al. Gaussian 09, Revision E.01, Gaussian, Inc, Wallingford CT; 2013. [71] R.G. Parr, P.K. Chattaraj, Principle of maximum hardness, J Am Chem Soc 113 (1991) 1854–2185. [72] E. Chamorro, P.K. Chattaraj, P. Fuentealba, Variation of the Electrophilicity Index along the Reaction Path, J. Phys. Chem. A 107 (2003) 7068–7072. [73] A. Hashmi, M.U. Farooq, I. Khan, J. Son, J. Hong, Ultra high capacity of hydrogen storage in Li decorated two dimensional C2N layer, J. Mater. Chem. A 00 (2016) 1–3. [74] V. Mahamiya, A. Shukla, B. Chkraborty, Ultrahigh reversible hydrogen storage in K and Ca decorated 4–6-8 Bi-Phenylene sheet, Int. J. Hydrogen Energy (2022), https://doi.org/10.1016/j0ijhydene.2022. [75] R.K. Sahoo, B. Chakraborty, S. Sahu, Reversible hydrogen storage on alkali metal (li and Na) decorated C20 fullerene: a density functional study, Int. J. Hydrogen Energy 46 (80) (2021) 40251–40261. [76] J. Niu, B.K. Rao, P. Jena, Interaction of H2 and He with metal atom, clusters and ions, Phys. Rev. Lett. 51 (1995) 4475–4484. [77] J. Niu, B.K. Rao, P. Jena, Binding of hydrogen molecules by a transition-metal ion, Phys. Rev. Lett. 68 (1992) 2277–2280. [78] D. Cremer, E. Kraka, Chemical bonds without bonding electron density — does the difference electron-density analysis suffice for a description of the chemical bond? Int. Ed. Engl. 23 (1984) 627. [79] E. Espinosa, I. Alkorta, J. Elguero, E. Molins, From weak to strong interactions: a comprehensive analysis of the topological and energetic properties of the electron density distribution involving X-H⋯F–Y systems, J. Chem. Phys 117 (2002) 5529. [80] U. Koch, P.L. Popelier, Characterization of CHO hydrogen bonds on the basis of the charge density, J. Phys. Chem. 99 (24) (1995) 9747–9754. [81] Guo C. and wang C. Stability and hydrogen storage properties of Mx-B6H6 complexes (M=Y-Mo, Ru-Ag, x=1-2)ACS Sustainable Chem. Eng. 2021, 9, 10868− 10881. [82] Y. Zhang, X. Han, X. Cheng, The high capacity hydrogen storage material of Y doped B40: a theoretical study, Chem Phys Lett. 739 (2020), 136961. [83] C. Guo, C. Wang, The effect of charge on di-hydrogen storage capacity of Sc2-C6H6, Int. J. Hydrogen Energy 46 (2020) 955–966. [84] A. Salehabadi, M.F. Umar, A. Ahmad, M.I. Ahmad, N. Ismail, M. Rafatullah, Carbon-based nanocomposites in solid-state hydrogen storage technology: an overview, Int. J. Energy Res. 44 (14) (2020) 11044–11058. [85] M. Mehrabi, P. Parvin, A. Reyhani, S.Z. Mortazavi, Hydrogen storage in multiwalled carbon nanotubes decorated with palladium nanoparticles using laser ablation/chemical reduction methods, Mater. Res. Express 4 (9) (2017), 095030. 10