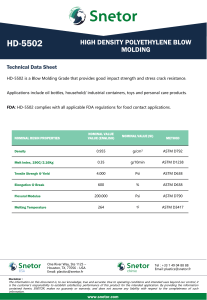
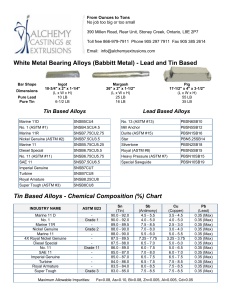
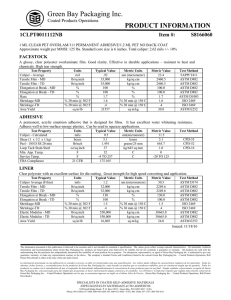
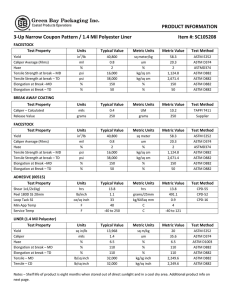
Interconversion Distillation 26 oil fractions (naphtha, kerosene, diesel) of different origins (different crude oils and different refining processes) with aromatics contents from 13 to 87 wt.% were analyzed for their distillation characteristics applying true boiling point (TBP - ASTM D-2892), ASTM D-2887 (simulated distillation = SD) and ASTM D-86 procedures. The methods of Daubert and Edmister were applied to convert ASTM D86 and ASTM D-2887 into TBP It was found that the simulated distillation itself was equivalent to TBP The average absolute deviation between SD and TBP for 96% of the investigated oil fractions was lower than that between two consecutive TBP analyses of the same oil fraction (FCC LCO; av.abs.dev. = 5.6 degrees C). ASTM method D86 This method is used for the distillation of motor gasoline’s, Kerosines aviation, gasolines, Aviation turbine fuels, naphtha’s, kerosene’s, gasoline , distillate fuel oils and similar petroleum products. It is carried out at atmospheric pressure. An exposed thermometer is used and temperature is reported without stem corrections. ASTM D86 distillations are plotted in volume percent while simulated distillations are plotted in weight percent. In this test, the sample is boiled and the vapors are collected and condensed as they are produced. Vapor temperatures are then recorded as a function of cumulative liquid volume collected. Because no reflux is used and there is only one equilibrium stage, the initial boiling point of this assay is larger and the final point is lower than the one in the TBP curve. ASTM Method D1160 This method is used for heavy petroleum products which can be vaporized partially or completely at a maximum liquid temperature of 750F at absolute pressure down to 1 mm hg and condensed as liquid as the pressure of the test. It is carried out at pressure between1 mm Hg and 760 mm Hg, Absolute. Thermocouple is used for temperature measurement. TBP distillation data obtained at one pressure may be converted to another pressure by procedure 3A4.1. ASTM distillation data may be similarly converted (as described in ASTM Method D1160) but with somewhat reduced reliability. The preferred procedure for converting ASTM distillation data taken at one pressure to that at another pressure is to obtain the corresponding TBP data, convert this by procedure 3A4.1 and then reconvert to ASTM distillation Data. The procedure is outlined for 10 mm Hg to 1 atmospheric Interconversion of distillation data for petroleum fractions at subatmospheric pressures Discussion The following procedure is recommended to convert ASTM or TBP distillation data between subatmospheric pressure (usualy 1, 10, 100 mm Hg) and between subatmospheric pressure and atmospheric pressure (760 mm Hg). Procedure A. Data at Subatmospheric Pressure Step 1: Assume the Watson k of the petroleum fraction is 12, and convert the data using procedure 5A1.13 Step 2: Since the Watson K is set at 12, no Watson K correction is necessary. B. Data at atmospheric pressure Step 1: If the specific gravity and mean average boiling point are known or can be calculated, determine the Watson K otherwise consider K=12 TBP The separation of hydrocarbons can be improved if the vapor and its condensates (reflux) are in intimate contact for some time during distillation and a reproducible distillation analysis is possible. Such a method of distillation is known as true boiling point (TBP) distillation. The true boiling point curve is basically a plot of the boiling point of each component of the mixture as a function of the cumulative volumetric fraction distilled. It implies that a batch distillation column with a large number of trays and a large reflux ratio is used, which will ensure that each component is separated at a time. In practice, the ASTM 8 D2892 test method (ASTM is an acronym for American Society for testing and Materials) is used. It consists of using a 15 to 18 tray distillation column operating at a 5:1 reflux ratio. The distilled volume is measured and the temperature in the reboiler is recorded. Conversion between ASTM and TBP TBP distillation is both tedious and time consuming in comparison with the ASTM method, there has been an intensive to develop correlation to convert ASTM to TBP distillation while at the same time achieving the benefits of the detailed separation of TBP with the Little effort of ASTM distillation. TBP distillation is both tedious and time consuming in comparison with the ASTM method, there has been an intensive to develop correlation to convert ASTM to TBP distillation while at the same time achieving the benefits of the detailed separation of TBP with the Little effort of ASTM distillation. Riazi and Daubert uses the interconversion, referred to as API method. TBP=a(ASTM D86)b Where, a and b are constants varying with percent of liquid samples distilled as in given table. TBP is true boiling point temperature at 0, 10,30,50,70,90, and 95 volume percent distilled, in degree Rankin.ASTM D86 is the observed ASTM D86 temperature at corresponding volume percent distilled, in degree Rankin. Index number I 1 2 3 4 5 6 7 Ai Bi 7.4012 4.9004 3.0305 0.8718 2.5282 3.0419 0.1180 0.6024 0.7164 0.8008 1.0258 0.8200 0.750 1.6606 Distillation ASTM D86 to TBP(Riazi Method) Volume Coefficient Coefficient Temperature Temperature 0 0 % a b D86 C TBP C Distilled 0 0.9177 1.0019 36.5 14.110 10 0.5564 1.0900 54.1 33.4 30 0.7617 1.0425 76.9 68.9 50 0.9013 1.0176 101.5 101.6 70 0.8821 1.0226 131.0 135.1 90 0.9552 1.0110 171.0 180.5 95 0.8177 1.0355 186.5 194.1 Interconversion of ASTM D86 TBP distillation curves at atmospheric pressure Following equations are used to interconvert ASTM D86 and atmospheric TBP distillation data TBP= a(ASTM D86)b ASTM D86= a(-1/b)(TBP)(1/b) Where a,b= are constant varying with percent of liquid sample distilled as given below Volume % distilled a b 0 0.9167 1.0019 10 0.5277 1.0900 30 0.7429 1.0425 50 0.8920 1.0176 70 0.8705 1.0226 90 0.9490 1.0110 95 0.8008 1.0355 TBP= True boiling point temperature at 0, 10, 30, 50, 70, 90 and 95 volume percent distyilled in 0R ASTM D86= observed ASTM D86 temperature at corresponding volume percent distilled in 0R Comment on procedure 3A1.1 Purpose The purpose of this procedure is to predict an atmospheric TBP distillation from an ASTM D86 distillation or to predict ASTM D86 data from an atmospheric TBP distillation. Limitations: As discussed in the introduction, occasional severe errors may arise from the use of this procedure especially at the initial point. Furthermore, it should be not for convertinh ASTM to TBP data for use with procedure 3C1.1 since the flash calculations of procedure 3C1.1 are highly sensitive to slight inaccuracies in the TBP curve Volume % Distilled ASTM D86 Temperature Range F TBP Temperature Range , F 0 73-599 -50-616 10 97-583 51-561 30 119-596 97-590 50 138-608 135-608 70 151-621 153-626 90 166-647 181-662 95 162-750 163-794 Reliability Difference between the estimated and experimental TBP and ASTM D86 temperature are as given below at various volume % distilled points Volume % Distilled Average Deviation, F 0 21 10 11.1 30 7.6 50 6.1 70 6.6 90 7.9 95 11