A dormancy mechanism limits metastasis of early spreading cancer cells
Anuncio

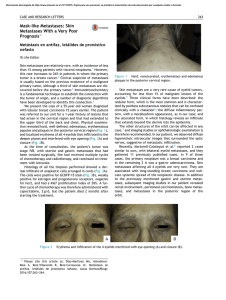
Research briefing A dormancy mechanism limits metastasis of early spreading cancer cells Mesenchymal-like and pluripotency-like programs coordinate the dissemination and long-lived dormancy of early breast cancer cells. The transcription factor ZFP281 controls these programs, preventing the acquisition of an epithelial-like proliferative phenotype and serving as a previously unrecognized barrier to metastasis. This is a summary of: Aguirre-Ghiso, J. A. et al. ZFP281 drives a mesenchymal-like dormancy program in early disseminated breast cancer cells that prevents metastatic outgrowth in the lung. Nat. Cancer https://doi.org/10.1038/s43018022-00424-8 (2022). Published online: 14 September 2022 Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. The question Cancer cells had long been believed to disseminate and metastasize only during late stages of cancer evolution. However, using genetic lineage trees, we have previously shown that disseminated cancer cells (DCCs) can start to colonize organs very early in cancer evolution. Early DCCs can remain dormant for long periods before forming overt metastases, sometimes without progressing through a primary tumor growth state, in parallel progression to the original primary tumor1. It is unclear whether this pause in progression is due to a slow evolution of early DCCs or to a mechanism that actively holds early DCCs in a dormant state before they proliferate and further evolve genetically. Understanding these mechanisms may enable the maintenance of early DCCs in this dormant state or selectively killing them before they metastasize. As early DCCs differ phenotypically from DCCs that are derived from late evolved primary tumors, this knowledge may afford therapeutic strategies that better target all potential metastasis-initiating cells. The discovery Early DCCs have not been isolated and molecularly and phenotypically characterized in depth1,2. We used mouse models and single-cell RNA sequencing to reveal the heterogeneity and plasticity of breast cancer DCCs that spread to the lungs across the evolution of mammary cancer. We found that early DCCs have very heterogeneous transcription programs, acquire different mesenchymal-like (M-like) phenotypes and express dormancy signatures in vivo. The M-like programs in different DCC clusters seemed to be driven by transcription factors such as those encoded by Neurod1, Sox9, Prrx1, Snai2 and Twist1, among others. Using epigenetic analysis and gain-of-function or loss-of-function assays, we further found that the transcription factor zinc finger protein 281 (Zfp281, encoded by ZNF281 in humans)3, which regulates primed pluripotency, is a key regulator of early DCC spread and dormancy. During primed pluripotency in the blastocyst, Zfp281 represses Nanog while enabling expression and function of Sox2 and Oct4 (also known as Pou5f1). In early cancer cells, Zfp281 drives the epithelial–mesenchymal transition (EMT) that enables dissemination. Accordingly, M-like clusters of DCCs were enriched for Oct4 and Sox2 target genes, whereas DCCs clusters acquiring more epithelial-like features and, therefore, beginning proliferation, showed enrichment for genes controlled by Nanog and Klf4. Nature Cancer | VOL 3 | October 2022 | 1147–1148 | www.nature.com/natcancer Zfp281 is induced by fibroblast growth factor 2 (Fgf2) and twist-related protein 1 (Twist1), suppresses proliferation in the primary tumor in the mammary fat pad and induces efficient dissemination to target organs. After dissemination, Zfp281 expression is maintained and holds early DCCs in a prolonged dormant, non-proliferative state via the induction of the class II cadherin 11 (encoded by Cdh11). We demonstrated that even aggressive primary tumor cells, with low or no Zfp281 expression, can be reprogrammed into dormancy and prevented from metastasizing by regaining Zfp281 (Fig. 1) or Cdh11 expression. Thus, Zfp281 regulates a dormancy program in early DCCs that precedes a slow proliferation phase towards metastasis and must be overridden for metastasis outgrowth. The implications This mechanism of DCCs dormancy goes beyond the classical view of the metastatic cascade. Our findings contribute to an improved understanding of breast cancer early dissemination and dormancy, and may also be expanded and tested in other cancers, especially those in which early spread has been proposed4. More work is needed to validate ZFP281 and the M-like dormancy program in human DCCs. However, detection of ZFP281 may help to measure the abundance of early DCCs in patients and the proclivity of these cells to develop recurrences. These findings may also enable the exploitation of these mechanisms to eliminate early dormant DCCs or force them into an asymptomatic phenotype, which reduces the chances of metastasis. Of note, our approach could not fully distinguish early DCCs from those spreading from late lesions and co-existing in the lungs. The plasticity of both early and late DCCs may preclude the identification of a unique marker to distinguish them. We also need to understand how hormonal regulation influences this process, as late recurrence is prevalent in hormone receptor-positive breast cancer. Additionally, we need to investigate how early ZFP281+ DCCs interact with late arriving and more evolved DCCs, which may cooperate in metastasis initiation5. Finally, whether early DCCs are the main orchestrators of the termed pre-metastatic niche5 should be explored. Julio A. Aguirre-Ghiso1 and Ana Rita Nobre2 Cancer Dormancy and Tumour Microenvironment Institute, Albert Einstein College of Medicine, Bronx, NY, USA. 2 Memorial Sloan Kettering Cancer Centre, New York, NY, USA. 1 1147 “ Expert opinion The topic is interesting, novel and with potential clinical relevance. The most compelling finding is the linking of EMT (previously known to be important for dissemination) with dormancy, References and the identification of a novel role for ZFP281, which was not previously implicated in cancer metastasis.” Neta Erez, Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel. Figure 2. Schardt, J. A. et al. Genomic analysis of single cytokeratin-positive cells from bone marrow reveals early mutational events in breast cancer. Cancer Cell 8, 227–239 (2005). This paper reveals that early cancer cells can spread in patients with pathologically confined breast cancer and hinted to an EMT as potentially involved in the process. b a DCIS PT ZFP-OE PT control 3. Fidalgo, M. et al. Zfp281 functions as a transcriptional repressor for pluripotency of mouse embryonic stem cells. Stem Cells 29, 1705–1716 (2011). This article shows that Zfp281 is a repressor of Nanog, thereby allowing for differentiation programs during embryogenesis, and enables the understanding of Zfp281 function in early dissemination. 0 µm 25 Fig. 1 | ZFP281 maintains dormancy of early DCCs. a, Human breast ductal carcinoma in situ (DCIS) lesion, showing early cancer cells positive for nuclear ZFP281 (in green), which indicates that these cells already have putative dissemination and pro-dormancy potential. Blue indicates DAPI, 4′,6-diamidino2-phenylindole. b, Advanced breast cancer cells expressing human epidermal growth factor receptor 2 (HER2) can efficiently form spontaneous metastasis (dark pink) in mouse lungs (top, control) but can be programmed into a dormant phenotype with reduced metastatic potential (bottom) when ZFP281 expression and function is restored. OE, overexpression; PT, primary tumor; scale bars, 2 mm. © 2022, Aguirre-Ghiso, J. A. et al. Behind the paper We hypothesized that drivers of early spread and dormancy had to be already active in the early lesions. At the beginning of this study, A.R.N. computationally mined RNA sequencing data from early lesions and identified ZFP281 as a transcription factor of elusive function. Luckily, Jianlong Wang, who was working at our institution at the time, is an expert on ZFP281 in embryogenesis, and we immediately started collaborating. This specific program was unfunded until very recently. Large National Institutes of Health 1148 1. Hosseini, H. et al. Early dissemination seeds metastasis in breast cancer. Nature 540, 552–558 (2016). This paper reveals that metastasis can be founded independently of the primary tumor by early DCCs in a subset of patients with HER2+ breast cancer. (NIH) team grants on early dissemination biology have been hard to obtain despite requests for applications based on our earlier work. The most satisfying experiments were the most uncertain; those for which we waited ~6 months for metastasis detection. These results then corroborated the singlecell sequencing data pointing to the fact that ZFP281 drives the M-like program of dissemination and dormancy of early DCCs and can reprogram malignant cells into dormancy. J.A.A.-G. 4. Klein, C. A. Cancer progression and the invisible phase of metastatic colonization. Nat. Rev. Cancer 20, 681–694 (2020). This review article provides a wellorchestrated and integrated view of metastatic progression, with an emphasis on what may happen during the time residual cancer persists undetected in patients. 5. Sosa, M. S., Bragado, P. & Aguirre-Ghiso, J. A. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat. Rev. Cancer 14, 611–622 (2014). This review article provides an integrated view of the mechanisms that control cancer cell dormancy across cancers and how this biology might be linked to early dissemination, pre-metastatic niche biology, stem-cell biology and immune regulation of cancer dormancy. From the editor “ By identifying the role of a key pluripotency factor in driving M-like gene expression programs to induce tumor dormancy, this study highlights the plastic manner in which aspects of pluripotency, differentiation, EMT and dormancy are orchestrated during early tumor cell dissemination.” Editorial Team, Nature Cancer. Nature Cancer | VOL 3 | October 2022 | 1147–1148 | www.nature.com/natcancer