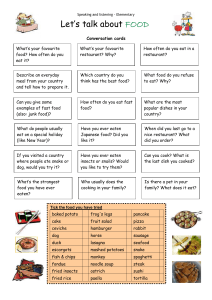
Snake Length-Mass Allometry: Estimating Mass from Length
Anuncio

See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/262872508 Length–mass allometry in snakes Article in Biological Journal of the Linnean Society · January 2013 DOI: 10.1111/j.1095-8312.2012.02001.x CITATIONS READS 78 1,520 2 authors: Anat Feldman Shai Meiri Tel Aviv University Tel Aviv University 29 PUBLICATIONS 3,477 CITATIONS 276 PUBLICATIONS 10,918 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: evolution, macroecology and biogeography of reptile traits View project evolution of island vertebrates - sizes syndromes etc. View project All content following this page was uploaded by Shai Meiri on 11 November 2020. The user has requested enhancement of the downloaded file. SEE PROFILE bs_bs_banner Biological Journal of the Linnean Society, 2013, 108, 161–172. With 2 figures Length–mass allometry in snakes ANAT FELDMAN* and SHAI MEIRI Department of Zoology, Tel Aviv University, 69978 Tel Aviv, Israel Received 31 May 2012; revised 5 July 2012; accepted for publication 5 July 2012 Body size and body shape are tightly related to an animal’s physiology, ecology and life history, and, as such, play a major role in understanding ecological and evolutionary phenomena. Because organisms have different shapes, only a uniform proxy of size, such as mass, may be suitable for comparisons between taxa. Unfortunately, snake masses are rarely reported in the literature. On the basis of 423 species of snakes in 10 families, we developed clade-specific equations for the estimation of snake masses from snout–vent lengths and total lengths. We found that snout–vent lengths predict masses better than total lengths. By examining the effects of phylogeny, as well as ecological and life history traits on the relationship between mass and length, we found that viviparous species are heavier than oviparous species, and diurnal species are heavier than nocturnal species. Furthermore, microhabitat preferences profoundly influence body shape: arboreal snakes are lighter than terrestrial snakes, whereas aquatic snakes are heavier than terrestrial snakes of a similar length. © 2012 The Linnean Society of London, Biological Journal of the Linnean Society, 2013, 108, 161–172. ADDITIONAL KEYWORDS: body mass – body size – microhabitat – mode of reproduction – shape – snout–vent length – total length – venomousness. INTRODUCTION An organism is an ensemble of many characteristics, among which body size is undoubtedly one of the most important. Body size is strongly related to the organism’s physiology, energy requirements, ecology and life history (Calder, 1984; Shine, 1994a; Brown et al., 2004). Size is therefore the focus of many ecological and evolutionary studies, dating back to the inspiring studies of Bergmann (1847; translation in James, 1970) and Cope (1887) and continuing to our time (e.g. Hutchinson & MacArthur, 1959; Foster, 1964; Griffiths, 2012). Greater accessibility to data has enhanced the compilation of size data for some of the major vertebrate taxa (Smith et al., 2003; Olden, Hogan & Zanden, 2007; Meiri, 2008; Olson et al., 2009), and these datasets have allowed us to search further for ecological and evolutionary processes involving size. The body size of snakes has been studied in a variety of contexts, such as geographical variation *Corresponding author. E-mail: [email protected] (e.g. Ashton & Feldman, 2003; Olalla-Tárraga, Rodrıguez & Hawkins, 2006; Terribile et al., 2009; Amarello et al., 2010), range size (e.g. Bonfim, DinizFilho & Bastos, 1998; Reed, 2003), insularity (Boback, 2003), body size frequency distributions (Cox, Boback & Guyer, 2011), optimality (Boback & Guyer, 2003) and natural history (Pough & Groves, 1983). The measure of size in these studies, and in others, was length [Olalla-Tárraga et al. (2006) converted all lengths to masses, but used the same equation for all species]. This is undoubtedly because the snout–vent length (SVL) and total length (TL) are the most common size measures reported for snakes. Mass, another measure of body size, is rarely reported, and thus is rarely used. Some authors have claimed that length is a more appropriate size proxy than mass (Boback, 2003; Boback & Guyer, 2003), because mass depends on factors such as breeding condition, season, health, and size of and time from the last meal (Meiri, 2010). Furthermore, length is probably highly correlated with mass (Kaufman & Gibbons, 1975; Guyer & Donnelly, 1990). However, being a linear measure, length may fail to explain variation in body shape (Meiri, 2010), and snakes, although all © 2012 The Linnean Society of London, Biological Journal of the Linnean Society, 2013, 108, 161–172 161 162 A. FELDMAN and S. MEIRI having elongated body forms (Lillywhite, 1987), are differently shaped (Pough & Groves, 1983; Aubret & Shine, 2009). Thus, length should be regarded carefully as a proxy for body size. Mass, however, is considered to be the best measure of body size in life history studies (Hedges, 1985). Mass is also the measure of choice in physiological studies (e.g. Seymor, 1987; Glazier, 2009; Bronikowski & Vleck, 2010), because various physiological rates (e.g. metabolic rate; Kleiber, 1947; Nagy, 2005) are mass dependent. Many ecological and physiological attributes vary allometrically with body mass (Peters, 1983; Schmidt-Nielsen, 1984). Hence, in many instances, mass will be a more useful proxy than length for body size. In addition, as lengths cannot be compared directly across groups of organisms that differ in shape, comparison between such groups that is based on length may prove to be misleading, but their masses can still be meaningfully compared (Hedges, 1985; Meiri, 2010). Because snake masses are rarely reported, they need to be estimated from the commonly reported measure, length. Pough (1980) presented the most comprehensive allometric equations for the estimation of snake mass from length. He proposed two general equations – one for the calculation of mass from SVL (mass = 6.6 ¥ 10-4 ¥ SVL3.02) and the other for the calculation of mass from TL (mass = 3.5 ¥ 10-4 ¥ TL3.02) (mass in grams, length in centimetres). However, Pough’s pioneering equations are based on the length and mass data of only 13 species of colubrids and vipers from the southern USA (data from Kaufman & Gibbons, 1975) and, as such, may not generalize to other snake taxa, if the latter have different body shapes. Vipers, for example, are generally more robust than other snakes (Pough & Groves, 1983; and see below), and so are constrictors (see below). Moreover, body shape and hence mass–length relationships are influenced by phylogenetic affinities, life history traits and ecological characteristics (Pough & Groves, 1983; Shine, 1994a; Martins et al., 2001; Brown et al., 2004). Arboreal species, for example, are generally more slender and have longer tails than terrestrial species (Guyer & Donnelly, 1990; Lillywhite & Henderson, 1993; Martins et al., 2001), and burrowing or aquatic species may differ even more from above-ground active snakes (Murphy, 2007; and see below). One equation for the estimation of mass ignores any such difference, and these differences may be too great to be neglected. More specific allometries will allow us to assess snake masses and the variation between different ophidian clades more accurately. Such equations have already been developed for lizards (Meiri, 2010; Pincheira-Donoso et al., 2011), and have been proven to be a better predictor of mass than is the single equation of Pough (Pincheira-Donoso et al., 2011). We compiled a database of 423 snake species in 10 families to develop new allometries for the estimation of snake mass from their SVL and TL, and evaluated the explanatory power of the equations depending on the measure used (SVL or TL). To overcome phylogenetic affinities and biological differences between clades, we generated different equations for different snake lineages. We then evaluated the predictive power of the equations and compared it with the predictive power of Pough’s allometric equations. Finally, we attempted to estimate the influence of phylogenetic, ecological and life history traits on the relationship between mass and length. We specifically estimated the influence of reproduction mode, venomousness (function-based definition of venom, i.e. ‘a toxic compound injected into prey or predator to cause rapid death or incapacitation’; Fry et al., 2012) and activity time on this relationship. As there is a relationship between microhabitat use and body shape (for example, arboreal species are lighter than terrestrial species; Lillywhite & Henderson, 1993; Martins et al., 2001), we added microhabitat use as a covariate in the model. We formulated four hypotheses: 1. Being elongated, snakes have limited abdominal space to hold their litter or eggs (Bonnet et al., 2000). Because viviparous species, unlike oviparous species, hold their embryos until the end of their development, we predicted that viviparous species would be heavier than oviparous species, because females need more abdominal space to retain their embryos. Moreover, the evolution of viviparity is associated, in some species, with a shift towards larger female size relative to male size (Shine, 1994b), indicating that viviparous species may be relatively heavier than oviparous species. 2. In congruence with former studies (e.g. Guyer & Donnelly, 1990; Martins et al., 2001), we hypothesized that mass would decrease on a gradient from terrestrial to arboreal snakes, enabling the latter to move more easily on trees. We further predicted that aquatic species would be heavier, on average, than others to better retain heat. 3. Body shape may play a significant role in heat transfer (e.g. Spotila et al., 1973). For example, assuming a cylindrical shape and a specific density of 1 g cm-3, a 1130-mm (TL) and 94.7 g Dolichophis jugularis from Israel [Tel Aviv University Zoological Museum (TAUM) specimen #10075] has a surface/volume ratio of 12.2 mm-1, whereas a heavier (432.2 g) Daboia palestinae (TAUM #8186) with the same length has a surface/volume ratio of © 2012 The Linnean Society of London, Biological Journal of the Linnean Society, 2013, 108, 161–172 SNAKE LENGTH–MASS ALLOMETRY 5.7 mm-1. Nocturnal species are not exposed to solar radiation, and the heat exchange between them and the environment is influenced by conduction and convection (Heatwole & Taylor, 1987). Because nocturnal species face, on average, lower temperatures than diurnal species, we predicted that nocturnal species would benefit from a lower surface to volume ratio (i.e. would be heavier than diurnal species) to retain heat and reduce the rate of cooling (Slip & Shine, 1988). 4. Venom in snakes facilitates prey manipulation, swallowing and prey handling (Kardong, 1979). Hence, nonvenomous snakes must invest more power in killing their prey, either by biting or constricting. We predicted that nonvenomous snakes would therefore be heavier than venomous snakes of a similar length as a result of the more strongly developed muscles needed for prey handling. METHODS DATA We accumulated data on length (SVL and TL, in millimetres) and body mass (in grams) for 423 snake species. Data are mainly from the published literature and from museum specimens housed at TAUM. We also measured live snakes at the Meier Segal’s Garden for Zoological Research, Tel Aviv University. We used only literature length and mass data that were reported together for a species in the same publication and referred to the same individual or individuals. Because sexual size dimorphism (SSD) is common in snakes (Seigel & Ford, 1987; Madsen & Shine, 1993; Shine et al., 1998) and may affect the mass–length relationships (Kaufman & Gibbons, 1975), we collected data separately for males, females and unsexed individuals. We did not use data for females that were known to be gravid at the time of measurements. All museum specimens used were weighed and measured prior to preservation. When several measurements were available for the same species, we used the mean values of all data for the species. However, we did not average mass data if they were associated with SVL data for some individuals and with TL data for others. Instead, we created two datasets per species – one for SVL and mass and the other for TL and mass. We used only data from adults. Taxonomy follows Uetz (2011). We classified species as either oviparous or viviparous and treated ovoviviparous species as viviparous, because females retain their eggs inside their bodies until hatching. We classified species as venomous or nonvenomous and treated opisthoglyphous (rearfanged) species as venomous (a preliminary analysis 163 found that our results are robust to treating them as venomous). We classified species as diurnal, nocturnal or cathemeral, and microhabitat preferences as aquatic (including semi-aquatics), burrowers (fossorial and semi-fossorial), terrestrial, terrestrial– arboreal and arboreal. We analysed this relationship for all snake clades together, controlling for phylogenetic affinities. We repeated the analysis for colubrids, a species-rich lineage which, unlike most snake families, contains much ecological and morphological diversity in the traits examined. DATA ANALYSIS Prior to statistical analysis, we log10-transformed all weight and length data. In a preliminary analysis for each family, we found that allometries based only on males did not differ from those based only on females (not shown). Therefore, we analysed all data (i.e. males, females and unsexed individuals) together. To develop family-specific equations, we regressed mass separately on SVL and on TL for each lineage. To compare the predictive power of the equations using SVL or TL, we used only species for which we had both SVL and TL data from the same individual/ individuals, and selected the best models according to the Akaike Information Criterion (AIC) (Wagenmakers & Farrell, 2004). To estimate the effect of ecological and life history traits on the relationship between mass and length, we used backwards-stepwise elimination and determined the best model as that in which all traits were statistically significant (at P = 0.05). To evaluate the predictive power of the allometries, we randomly divided our data, using the ‘rand’ command in Microsoft Excel, into two, and developed allometries for the relationship between mass and SVL for each half. We then used these allometries to calculate the mass of the other half. We also calculated, for each species, the estimated mass using Pough’s equation, and tested for the difference and percentage of deviation between the original mass, our predicted mass and Pough’s predicted mass. All statistical tests were conducted using R 2.13.1 (R Development Core Team, 2011). PHYLOGENY To control for the effects of shared ancestry, we assembled a species-level phylogeny from published phylogenetic trees. The higher level branching (e.g. family and subfamily levels) is based on Lee et al. (2007) and Pyron et al. (2011) for Alethinophidia (‘advanced snakes’) and on Vidal et al. (2010) for Scolecophidia (‘blind snakes’). As several phylogenetic hypotheses have been suggested recently, we © 2012 The Linnean Society of London, Biological Journal of the Linnean Society, 2013, 108, 161–172 164 A. FELDMAN and S. MEIRI also analysed the data following the higher level tree of Vidal et al. (2007) to examine whether the results of our phylogenetic analysis are robust to the phylogenetic hypothesis we used. The tree of Vidal et al. (2007) differs from that of Pyron et al. (2011) in its interpretation of the branching of several Alethinophidian clades, especially within the superfamily Colubridea. To compare the models based on these two phylogenies, we used only species for which we had all ecological and life history data. Preliminary analysis yielded qualitatively the same results (in terms of the predictors in the best model and their direction), and thus we present here only the results of the model which is based on the phylogeny of Lee et al. (2007) and Pyron et al. (2011). The results based on Vidal et al.’s (2007) tree are presented in Appendix S3. Lacking branch lengths for most of the trees, we scaled branches to make the trees ultrametric using the cladogram transform in FigTree (Rambaut, 2010). We used phylogenetic generalized least-square (PGLS) regression to account for phylogenetic nonindependence. We adjusted the strength of phylogenetic nonindependence using the maximum likelihood value of the scaling parameter l (Pagel, 1999) implemented in the R package CAIC (Orme, online, http://r-forge.r-project.org/projects/caic/). The scaling parameter l varies between zero (no phylogenetic signal) and unity (strong phylogenetic signal, equivalent to phylogenetically independent contrast analysis). RESULTS Our dataset (Appendix S1) includes 423 species (336 species for SVL and 337, partially overlapping species for TL). It contains both the smallest known snake (Tetracheilostoma carlae, SVL = 93.7 mm, TL = 99.4 mm, 0.6 g; Hedges, 2008) and a few of the largest species (green anacondas, reticulated, Burmese and African rock pythons, up to SVL = 3370 mm, TL = 3735 mm and 21.75 kg in our database). We calculated family-specific allometric equations for all Alethinophidian families with N ⱖ 6 species for which we had length and mass data. The infra-order Alethinophidia is represented in our data by seven families and the infra-order Scolecophidia is represented by three (however, because of the small sample size, we grouped all Scolecophidians and generated one equation for the entire clade). The family mass–length allometries are shown in Table 1. Analysis of covariance revealed that intercepts differed significantly between families, both in the Table 1. Mass–length allometries for snake families and the infra-order Scolecophidia Family Measure N Slope SE 95% confidence interval Intercept SE R2 All SVL TL 336 337 2.786 2.597 0.063 0.073 2.661 2.452 2.910 2.744 -5.773 -5.465 0.172 0.208 0.853 0.789 PGLM SVL TL 241 235 2.578 2.443 0.079 0.092 2.422 2.261 2.734 2.625 -5.148 -4.989 0.254 0.313 0.834 0.777 Boidae SVL TL 15 13 2.776 2.856 0.279 0.331 2.171 2.126 3.380 3.585 -5.500 -5.886 0.814 0.982 0.883 0.871 Colubridae SVL TL 166 154 2.520 2.340 0.088 0.098 2.346 2.146 2.693 2.534 -5.143 -4.912 0.239 0.278 0.834 0.792 Elapidae SVL TL 26 49 2.453 2.407 0.215 0.188 2.009 2.028 2.897 2.786 -4.892 -4.819 0.606 0.539 0.844 0.777 Homalopsidae SVL TL 11 8 3.631 3.617 0.792 0.767 1.837 1.738 5.425 5.496 -7.713 -8.027 2.128 2.130 0.700 0.787 Lamprophiidae SVL TL 35 31 3.232 2.821 0.201 0.229 2.823 2.351 3.641 3.290 -7.092 -6.286 0.542 0.646 0.886 0.839 Pythonidae SVL TL 6 12 2.630 2.611 0.301 0.260 1.793 2.031 3.467 3.190 -5.124 -5.131 0.972 0.848 0.950 0.909 Viperidae SVL TL 60 51 2.655 2.910 0.107 0.156 2.440 2.596 2.869 3.223 -5.165 -6.013 0.293 0.438 0.913 0.877 Infra-order Scolecophidia SVL TL 17 18 2.985 3.068 0.553 0.489 1.804 2.031 4.165 4.104 -6.381 -6.596 1.254 1.144 0.660 0.711 PGLM, phylogenetic general linear model; SE, standard error; SVL, snout–vent length; TL, total length. Log mass/log length allometries for different snake families and infra-order Scolecophidia. © 2012 The Linnean Society of London, Biological Journal of the Linnean Society, 2013, 108, 161–172 SNAKE LENGTH–MASS ALLOMETRY Table 2. Amount of variation explained and Akaike Information Criterion (AIC) scores for allometries of mass on snout–vent length (SVL) and total length (TL) Family Measure R2 AIC All (245) SVL TL 0.837 0.773 94.555 175.774 All (corrected for family) SVL TL 0.881 0.858 29.100 72.263 Boidae (10) SVL TL 0.917 0.880 5.378 8.691 Colubridae (128) SVL TL 0.822 0.774 23.942 54.344 Elapidae (12) SVL TL 0.529 0.562 11.787 10.902 Homalopsidae (6) SVL TL 0.799 0.806 3.730 3.531 Lamprophiidae (30) SVL TL 0.886 0.839 -6.566 3.773 Viperidae (44) SVL TL 0.905 0.899 -27.145 -24.068 Infra-order Scolecophidia (15) SVL TL 0.634 0.622 11.982 12.454 Number of species parentheses. in each category is given Table 3. Parameter estimates for nonphylogenetic model. Slope and intercept values for oviparous, diurnal and terrestrial species in each family. The intercept value differences should be added for the calculation of other traits [for example, the intercept of a viviparous (0.069), nocturnal (–0.057) and arboreal (–0.312) viperid is –5.110 + 0.069 – 0.057 – 0.312] Intercept Slope Colubridae Boidae Elapidae Homalopsidae Lamprophiidae Pythonidae Viperidae Scolecophidia Viviparous Cathemeral Nocturnal Aquatic Arboreal Burrower Terrestrial-arboreal Intercept difference 2.668 -5.510 -5.072 -5.459 -5.324 -5.552 -5.177 -5.110 -5.642 0.069 -0.030 -0.057 0.163 -0.312 0 -0.068 in relationship between mass and SVL (F1,7 = 22.466, P << 0.001, Fig. 1) and in the relationship between mass and TL (F1,7 = 31.197, P << 0.001). Family slopes differed in the relationship between mass and TL (F1,7 = 2.101, P = 0.043), but not in the relationship between mass and SVL (F1,7 = 1.665, P = 0.117). Mass was usually explained better by SVL than by TL (Table 2). When the same individuals were used to derive allometric equations for both length measures, only in elapids and, to a lesser extent, in homalopsids, did TL explain more of the variation in mass than SVL. Because SVL is generally a superior predictor for mass (Table 2), we used SVLs to examine the factors influencing the length–mass relationship. FACTORS 165 INFLUENCING THE LENGTH–MASS RELATIONSHIP Nonphylogenetic analysis The nonphylogenetic model included 336 species (Appendix S1). The best model for snake mass included SVL (slope = 2.668, SE = 0.066), family (Fig. 1), microhabitat use, reproduction mode (Fig. 2) and diel activity. The model explained 92.7% of the variation in mass. There were no interactions between SVL and any parameter. In colubrids viviparous species were heavier than oviparous species, but this difference was marginally nonsignificant (intercept difference = 0.069, t = 1.761, P = 0.0793). Diurnal species were heavier than nocturnal species, but this difference was also marginally nonsignificant (intercept difference = 0.057, t = 1.815, P = 0.070). Shape was strongly correlated with microhabitat use, with aquatic species significantly heavier than all other snakes (P << 0.001 in all cases) and arboreal species significantly lighter than others (P << 0.001 in all cases). The difference between terrestrial and terrestrial–arboreal species was marginally nonsignificant (P = 0.095), and both terrestrial and terrestrial–arboreal species did not differ significantly from burrowing species. Parameter estimates of the full model are shown in Table 3. Viviparous colubrids were heavier, for their lengths, than oviparous colubrids (intercept difference = 0.143, t = 2.341, P = 0.02), and diurnal species were heavier than nocturnal species (intercept difference = 0.089, t = 2.346, P = 0.02). Differences concerning venomousness (intercept difference = 0.04, t = 1.250, P = 0.21) and microhabitat use (aquatic species being heavier than others and arboreal species being lighter than others, F1,4 = 14.435, P << 0.001) were qualitatively similar to those found in the previous analysis. This model explained 89.9% of the variation in colubrid mass. © 2012 The Linnean Society of London, Biological Journal of the Linnean Society, 2013, 108, 161–172 166 A. FELDMAN and S. MEIRI Figure 1. A, Mass–snout to vent length (SVL) relationship for snake families in our dataset. B, Mass–SVL relationship for snake families. All species were fitted with parameter estimates for a terrestrial, diurnal and oviparous snake. See Table 3 for parameter estimates. © 2012 The Linnean Society of London, Biological Journal of the Linnean Society, 2013, 108, 161–172 SNAKE LENGTH–MASS ALLOMETRY 167 Figure 2. A, Mass–snout to vent length (SVL) relationship for oviparous and viviparous snake species in our dataset: white, oviparous species; black, viviparous species. B, Mass–SVL relationship for oviparous and viviparous colubrids and viperids species. All species were fitted with parameter estimates for a terrestrial and diurnal snake. See Table 3 for parameter estimates. © 2012 The Linnean Society of London, Biological Journal of the Linnean Society, 2013, 108, 161–172 168 A. FELDMAN and S. MEIRI Table 4. Parameter estimates for phylogenetic model. Slope and intercept values for oviparous species in different microhabitats. The value of 0.118 should be added to the intercept values for the calculation of the intercept value of viviparous species in each microhabitat (for example, the intercept value of a viviparous arboreal species is -5.699 + 0.118) Intercept Slope Arboreal Aquatic Burrower Terrestrial–arboreal Terrestrial Viviparous Intercept difference 2.677 -5.699 -5.326 -5.329 -4.488 -5.451 0.118 Phylogenetic model The phylogenetic model included mass–SVL data for 241 species (Appendix S2). The maximum likelihood value of l (0.557) was significantly different from both zero and unity (P << 0.001). The best model for mass included SVL (slope = 2.677, SE = 0.074), mode of reproduction (viviparous species heavier than oviparous species; intercept difference = 0.118, P = 0.015) and microhabitat use, with arboreal species lighter than others (P << 0.001 in all cases) and burrowing and aquatic species heavier than terrestrial (P = 0.018 and 0.05, respectively) and terrestrial–arboreal (P = 0.01 and 0.03, respectively) species. Activity time was not correlated with mass, although the tendency was similar to that of the nonphylogenetic model (i.e. nocturnal species were lighter than diurnal species). The model accounted for 86.9% of the variation in snake mass. There were no interactions between SVL and other factors. Parameter estimates of the best phylogenetic model are shown in Table 4. Predictive ability of the equations We calculated mass for 302 species. Our calculated mass did not differ significantly from the real mass (paired t-test, t = 0.466, P = 0.641, see Appendix S4 for detailed results), whereas the mass calculated using Pough’s equation did (paired t-test, t = -13.052, P << 0.001). The mass predicted by our equations deviated from the actual mass by 13.53%, on average, vs. a mean deviation of 19.83% with Pough’s equation. DISCUSSION SVL and TL are the two most common measures for snake body size, and both are used in ecological and evolutionary studies of snake body size. Snake mass is seldom reported in herpetological, ecological and evolutionary studies. This is despite its probability of being a better predictor for body size and a measure which allows comparisons among taxa with different body shapes (Hedges, 1985; Meiri, 2010). Here, we developed clade and family allometric equations for the calculation of mass from SVL and TL, and found that the former is generally a better predictor of mass (Table 2). This is somewhat paradoxical, because TL measures more of the animal than does SVL. Among legless squamates, snakes have relatively short tails (the ‘short-tailed burrowing morph’; Wiens, Brandley & Reeder, 2006; Sites, Reeder & Wiens, 2011). Nonetheless, relative tail length varies among species. Tail lengths can vary greatly between individuals of the same species (for example, male snakes have longer tails than females; Kaufman & Gibbons, 1975), between species inhabiting different microhabitats (Guyer & Donnelly, 1990; Martins et al., 2001) and between lineages. Thus, we attribute the differences in the predictive ability to a high variation in tail length between species with similar SVLs. Blindsnakes, a lineage of burrowing snakes, have distinctively short tails (Wiens et al., 2006; O’Shea, 2007), and this explains the similar predictive ability of SVL and TL in this lineage. Colubrids, however, have long tails (on average, ~30% of SVL in our data), and their tail length/SVL ratio differs greatly between species. The SVLs of colubrids active in different microhabitats are different (F4,116 = 9.08, P << 0.001), but those of terrestrial (N = 64, mean SVL of 488 mm) and arboreal (N = 14, mean SVL of 653 mm) colubrids are quite similar [Tukey’s honestly significant difference (HSD) test, P = 0.18], but the former have significantly shorter tails (127 mm vs. 270.6 mm, 26% vs. 41% of SVL; Tukey’s HSD test, P << 0.001). The differences in body shape between families are substantial (note the R2 gains when we correct for family, Table 2). This suggests that the equations of Pough (1980) may be too general because they do not consider phylogenetically and ecologically related body shape differences between lineages. Furthermore, the 95% confidence interval of the slope found for all snakes in our study (for both SVL and TL, Table 1) is entirely below three (indicating that long snakes are more slender than short snakes) and does not incorporate the slope of 3.02 of Pough. It is thus not surprising that the masses calculated by the application of Pough’s equations to our SVL data were significantly different from the actual masses of these snakes (Appendix S4). Mass and hence body shape are influenced by factors other than length. Phylogeny, natural history and ecology are undoubtedly important drivers of shape variation (França et al., 2008). In addition to © 2012 The Linnean Society of London, Biological Journal of the Linnean Society, 2013, 108, 161–172 SNAKE LENGTH–MASS ALLOMETRY phylogeny, we found that the mode of reproduction and microhabitat use and, to a lesser extent, diel activity play an important role in the relationship between mass and length. Our specific parameter estimates (Table 3) enable the calculation of the mass of a snake with known traits. For example, an oviparous, diurnal, terrestrial colubrid (e.g. Liophisreginae, SVL = 438.5 mm; Appendix S1) is expected to weigh approximately 34.6 g (log mass = 2.668 ¥ log SVL – 5.510), whereas a viviparous, diurnal, terrestrial viperid of roughly the same length (e.g. Crotalus pricei, SVL = 421.3 mm; Appendix S1) is predicted to weigh about 91.4 g (log mass = 2.668 ¥ log SVL – 5.110 + 0.069). An oviparous, burrower, nocturnal lamprophiid species of similar SVL, such as Atractaspis irregularis (SVL = 405 mm, Appendix S1), is predicted to weigh just 22.2 g (log mass = 2.668 ¥ log SVL – 5.552 – 0.057). The relationship between snake microhabitat use and body shape is well known (Pough & Groves, 1983; Guyer & Donnelly, 1990; see above). In agreement with former studies (Lillywhite & Henderson, 1993; Martins et al., 2001; França et al., 2008), we found that arboreal species are relatively lighter for their lengths than terrestrial or aquatic species. This shift in body form from short-tailed, heavy bodied snakes to long-tailed slender snakes may reflect the need of arboreal snakes to move on light branches and to manoeuvre across vegetation gaps (Lillywhite & Henderson, 1993; Martins et al., 2001). The long tail of arboreal forms may be an adaptation to greater manoeuvring ability among branches (Lillywhite & Henderson, 1993). Such a long tail is not required for terrestrial locomotion, and terrestrial species probably require the extra musculature for increased locomotion and prey handling efficiency. The (all aquatic) Homalopsid snakes have long and narrow forebodies and bulky posteriors (Murphy, 2007). Sea snake bodies (Elapidae: Hydrophiinae) are high for their sizes (Brischoux & Shine, 2011). This shape probably helps aquatic species to stabilize their bodies in the water and to facilitate locomotion in water (Murphy, 2007; Brischoux & Shine, 2011). We suggest that the robustness of aquatic species may also contribute to heat retention in a medium with high levels of heat conductivity. This assumption is supported by the findings of Spotilaa et al. (1973) that, the heavier a reptile, the less its body temperature fluctuates (whereas they found that length had a minor effect on body temperature). Contrary to our prediction, we found no difference between venomous and nonvenomous snakes. Although this result may indeed reflect reality, we assume that it may well result from different cladespecific body shapes. Viperids and elapids are all venomous, but viperids are heavier than elapids of 169 similar lengths (the viperid slope is steeper, and the two family lines intersect at 22.5 mm, way below the minimum length of snakes; Table 1, Fig. 1). We attribute this to their different foraging strategies. Most elapids are active hunters, whereas most viperids are ambush predators (Greene, 1997). Foraging mode may well be an important factor determining snake shape, with active foragers lighter than ambush predators (Greene, 1997; Ford & Hampton, 2009), perhaps in order to reduce the costs of locomotion. Furthermore, differences in body size and in mass–length relationship may also result from selection on other traits, such as on gape size, or reflect adaptations to hunt different prey types (Martins et al., 2001; Pyron & Burbrink, 2009). Nocturnal snakes are generally lighter than diurnal species, contradicting our hypothesis (and mirroring the pattern found for lizards; Meiri, 2010). We predicted that nocturnal species would be heavier than diurnal species for the purpose of better retaining heat. This result, however, may indicate that the importance of heat absorption is higher than the gain of heat retention. However, because we have no data on the thermal environment to which the species are exposed (e.g. temperature, solar radiation), this conclusion should be taken with caution. In congruence with our hypothesis, we found that viviparous species are heavier than oviparous species (Fig. 2). We attribute this to the differences in the development stage of the eggs and the period of time needed to retain the eggs. Being elongated, snakes have limited abdominal space to hold their litter or eggs (Bonnet et al., 2000). Oviparous species usually retain their eggs for less than 50% of the embryonic development (Shine, 1983; DeMarco, 1993), and eggs absorb water and become larger and heavier after they are laid (Qualls & Shine, 1995). Viviparous species, however, retain their eggs until full development, and may thus require more abdominal space for their developing young. Length is considered by some authors (e.g. Boback, 2003; Boback & Guyer, 2003) to be a better proxy than mass for snake size, but length data alone may fail to provide any significant information about the species biology or life history characters (Vitt, 1987). Others argue that the high correlation between the two makes them equally valuable (Kaufman & Gibbons, 1975; Guyer & Donnelly, 1990); however, as shown here, these correlations are influenced by phylogeny, ecology and life history traits. We suggest, however, that mass, and not length, is a superior proxy for size in most ecological and evolutionary contexts regarding snake body size, especially when comparing taxa that differ in shape. If weights are estimated from length using specific equations, such as those developed here (Tables 1 and 3), rather than © 2012 The Linnean Society of London, Biological Journal of the Linnean Society, 2013, 108, 161–172 170 A. FELDMAN and S. MEIRI from actual body masses, body condition, reproductive condition and feeding will not affect mass estimates. We show here that different snake clades have different allometries for the relationship between mass and length. These allometries reflect the differences in body shape that we attribute to phylogeny as well as to ecological and life history traits. We believe that the use of length as a measure for comparison between clades or between ecological and life history traits may poorly reflect reality. For example, oviparous (N = 214) and viviparous (N = 104) snakes have similar SVLs (529 and 526 mm, respectively), and thus similar masses, when mass is predicted by Pough’s equation (104 g vs. 106 g, t = 0.085, P = 0.932). Viviparous species, however, are heavier than oviparous species, both when we compare their actual masses (103.5 g vs. 52.4 g) and the masses calculated using our equations (96.8 g vs. 56.1 g; calculated mass: t = –2.711, P = 0.007; actual mass: t = –3.619, P < 0.0001). This result is congruent with the tendency found (Fig. 2) for viviparous species to be heavier than oviparous species of the same SVL. Although mass is an extremely important measure of snake biology, mass data are still seldom reported. We thus urge researchers to publish mass data as much as possible, especially for the less studied clades (e.g. Uropeltidae, Tropidophiidae). Using mass rather than length in ecological and evolutionary studies is likely to prove highly valuable, and increase our ability to arrive at meaningful conclusions across lineages. ACKNOWLEDGEMENTS We thank Erez Maza for great help with collection of the data from the Tel Aviv University Natural History Museum. Erez also helped us, together with Barak Levi, to measure live snakes. We gratefully thank Marinus Hoogmoed, Tiffany Doan, Jossehan Galúcio da Frota, Gleomar Fabiano Maschio, Alessandro Costa Menks Dale Nimmo and Otavio Marques for providing us with invaluable data. Three anonymous reviewers contributed helpful comments on an earlier version of this paper. REFERENCES Amarello M, Novak EM, Taylor EN, Schuett GW, Repp RA, Rosen PC, Hardy DL. 2010. Potential environmental influence on variation in body size and sexual size dimorphism among Arizona populations of the western diamondbacked rattlesnake (Crotalus atrox). Journal of Arid Environments 74: 1443–1449. Ashton KG, Feldman CR. 2003. Bergmann’s rule in nonavian reptiles: turtles follow it, lizards and snakes reverse it. Evolution 57: 1151–1163. Aubret F, Shine R. 2009. The origin of evolutionary innovations: locomotor consequences of tail shape in aquatic snakes. Functional Ecology 22: 317–322. Boback SM. 2003. Body size evolution in snakes: evidence from island population. Copeia 2003: 81–94. Boback SM, Guyer C. 2003. Empirical evidence for an optimal body size in snakes. Evolution 3: 195–211. Bonfim FS, Diniz-Filho JAF, Bastos RP. 1998. Spatial patterns and the macroecology of South American viperid snakes. Revista Brasileira de Biologia 58: 97–103. Bonnet X, Naulleau G, Shine R, Lourdais O. 2000. Reproductive versus ecological advantages to larger body size in female snakes, Vipera aspis. Oikos 89: 509–518. Brischoux F, Shine R. 2011. Morphological adaptations to marine life in snakes. Journal of Morphology 272: 566– 572. Bronikowski A, Vleck D. 2010. Metabolism, body size and life span: a case study in evolutionarily divergent populations of the garter snake (Thamnophis elegans). Integrative and Comparative Biology 50: 880–887. Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. 2004. Toward a metabolic theory of ecology. Ecology 85: 1771–1789. Calder WA. 1984. Size, function and life history. Cambridge, MA: Harvard University Press. Cope ED. 1887. The origin of the fittest. New York: Appleton. Cox CL, Boback SM, Guyer C. 2011. Spatial dynamics of body size frequency distributions for North American squamates. Evolutionary Biology 38: 453–464. DeMarco V. 1993. Estimating egg retention times in sceloporine lizards. Journal of Herpetology 27: 453–458. Ford NB, Hampton PM. 2009. Ontogenetic and sexual differences in diet in an actively foraging snake, Thamnophis proximus. Canadian Journal of Zoology 87: 254– 261. Foster JB. 1964. Evolution of mammals on islands. Nature 202: 234–235. França FGR, Mesquita DO, Nogueira CC, Araujo AFB. 2008. Phylogeny and ecology determine morphological structure in a snake assemblage in the central Brazilian Cerrado. Copeia 2008: 23–28. Fry BG, Casewell NR, Wüster W, Vidal N, Young B, Jacksone TNW. 2012. The structural and functional diversification of the Toxicofera reptile venom system. Toxicon 60: 434–448. Glazier DS. 2009. Ontogenetic body-mass scaling of resting metabolic rate covaries with species-specific metabolic level and body size in spiders and snakes. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology 153: 403–407. Greene HW. 1997. Snakes – the evolution of mystery in nature. Berkeley, CA: University of California Press. Griffiths D. 2012. Body size distributions in North American freshwater fish: large-scale factors. Global Ecology and Biogeography 21: 383–392. Guyer C, Donnelly MA. 1990. Length–mass relationships among an assemblage of tropical snakes in Costa Rica. Journal of Tropical Ecology 6: 65–76. © 2012 The Linnean Society of London, Biological Journal of the Linnean Society, 2013, 108, 161–172 SNAKE LENGTH–MASS ALLOMETRY Heatwole HF, Taylor J. 1987. Thermal ecology. Ecology of reptiles. Chipping Norton: Surrey Beatty & Sons, 21–96. Hedges SB. 1985. The influence of size and phylogeny on life history variation in reptiles: a response to Stearns. The American Naturalist 126: 258–260. Hedges SB. 2008. At the lower size limit in snakes: two new species of threadsnakes (Squamata: Leptotyphlopidae: Leptotyphlops) from the Lesser Antilles. Zootaxa 1841: 1–30. Hutchinson GE, MacArthur RH. 1959. A theoretical ecological model of size distributions among species of animals. American Naturalist 93: 117–125. James FC. 1970. Geographic size variation in birds and its relationship to climate. Ecology 51: 365–390. Kardong KV. 1979. ‘Protovipers’ and the evolution of snake fangs. Evolution 33: 433–443. Kaufman GA, Gibbons JW. 1975. Weight–length relationships in thirteen species of snakes in the southeastern United States. Herpetologica 31: 31–37. Kleiber M. 1947. Body size and metabolic rate. Physiological Review 27: 511–541. Lee MSY, Hugall AF, Lawson R, Scanlon JD. 2007. Phylogeny of snakes (Serpentes): combining morphological and molecular data in likelihood, Bayesian and parsimony analyses. Systematics and Biodiversity 5: 371–389. Lillywhite HB. 1987. Temperature, energetic, and physiological ecology. In: Seigal RA, Collins JT, Novak SS, eds. Snakes: ecology and evolutionary biology. New York: Macmillan Publishing Company, 422–477. Lillywhite HB, Henderson RW. 1993. Behavioral and functional ecology of arboreal snakes. In: Seigel RA, Collins JT, eds. Snakes: ecology and behavior. New York: McGraw-Hill, 1–48. Madsen T, Shine R. 1993. Phenotypic plasticity in body sizes and sexual size dimorphism in European grass snakes. Evolution 47: 321–325. Martins M, Araujo MS, Sawaya RJ, Nunes R. 2001. Diversity and evolution of macrohabitat use, body size and morphology in a monophyletic group of Neotropical pitvipers (Bothrops). Journal of Zoology 254: 529–538. Meiri S. 2010. Length–weight allometries in lizards. Journal of Zoology 281: 218–226. Murphy JC. 2007. Homalopsid snakes: evolution in the mud. Malabar, FL: Kreiger Publishing Company. Nagy KA. 2005. Field metabolic rate and body size. The Journal of Experimental Biology 208: 1621–1625. O’Shea M. 2007. Boas and pythons of the world. London: New Holland Publishers. Olalla-Tárraga MA, Rodrıguez MA, Hawkins BA. 2006. Broad-scale patterns of body size in squamate reptiles of Europe and North America. Journal of Biogeography 33: 781–793. Olden JD, Hogan ZS, Zanden MJV. 2007. Small fish, big fish, red fish, blue fish: size-biased extinction risk of the world’s freshwater and marine fishes. Global Ecology and Biogeography 16: 694–701. Olson V, Davies RG, Orme CDL, Thomas GH, Meiri S, Blackburn TM, Gaston KJ, Owens IPF, Bennet PM. 171 2009. Global biogeography and ecology of body size in birds. Ecology Letters 12: 249–259. Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401: 877–884. Peters HR. 1983. The ecological implications of body size. New York: Cambridge University Press. Pincheira-Donoso D, Fox SF, Scolaro JA, Ibargüengoytía N, Acosta JC, Corbalán V, Medina M, Boretto J, Villavicencio HJ, Hodgson DJ. 2011. Body size dimensions in lizard ecological and evolutionary research: exploring the predictive power of mass estimation equations in two Liolaemidae radiations. The Herpetological Journal 21: 35–42. Pough FH. 1980. The advantage of ectothermy for tetrapods. The American Naturalist 115: 92–112. Pough FH, Groves JD. 1983. Specialization of the body form and food habits of snakes. American Zoologist 23: 443–454. Pyron RA, Burbrink FT. 2009. Body size as a primary determinant of ecomorphological diversification and the evolution of mimicry in the lampropeltinine snakes (Serpentes: Colubridae). Journal of Evolutionary Biology 22: 2057– 2067. Pyron RA, Burbrink FT, Colli GR, de Oca ANM, Vitt LJ, Kuczynski CA, Wiens JJ. 2011. The phylogeny of advanced snakes (Colubroidea), with discovery of a new subfamily and comparison of support methods for likelihood trees. Molecular Phylogenetics and Evolution 58: 329–342. Qualls CP, Shine R. 1995. Maternal body-volume as a constraint on reproductive output in lizards: evidence from the evolution of viviparity. Oecologia 103: 73–78. R Development Core Team. 2011. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Rambaut A. 2010. FigTree, version 1.3.1. Edinburgh: Institute of Evolutionary Biology, University of Edinburgh. Reed RN. 2003. Interspecific patterns of species richness, geographic range size, and body size among new world venomous snakes. Ecography 26: 107–117. Schmidt-Nielsen K. 1984. Scaling. Why is animal size so important? Cambridge: Cambridge University Press. Seigel RA, Ford NB. 1987. Reproductive ecology. In: Seigal RA, Collins JT, Novak SS, eds. Snakes: ecology and evolutionary biology. New York: Macmillan Publishing Company, 210–252. Seymor RS. 1987. Scaling of cardiovascular physiology in snakes. American Zoologist 27: 97–109. Shine R. 1983. Reptilian reproductive modes: the oviparity– viviparity continuum. Herpetologica 39: 1–8. Shine R. 1994a. Allometric patterns in the ecology of Australian snakes. Copeia 1994: 851–867. Shine R. 1994b. Sexual size dimorphism in snakes revisited. Copeia 1994: 326–346. Shine R, Harlow PS, Keogh JS, Boeadi. 1998. The allometry of life-history traits: insights from a study of giant snakes (Python reticulatus). Journal of Zoology 244: 405– 414. Sites JW, Reeder TW, Wiens JJ. 2011. Phylogenetic insights on evolutionary novelties in lizards and snakes: © 2012 The Linnean Society of London, Biological Journal of the Linnean Society, 2013, 108, 161–172 172 A. FELDMAN and S. MEIRI sex, birth, bodies, niches, and venom. Annual Review of Ecology, Evolution, and Systematics 42: 227–244. Slip DJ, Shine R. 1988. Thermoregulation of free ranging diamond pythons Morelia spilota (Serpentes, Boidae). Copeia 1998: 984–995. Smith FA, Lyons SK, Ernest SKM, Jones KE, Kaufman DM, Dayan T, Marquet PA, Brown JH, Haskell JP. 2003. Body mass of late Quaternary mammals. Ecology 84: 3403. Spotila JR, Lommen PW, Bakken GS, Gates DM. 1973. A mathematical model for body temperatures of large reptiles: implications for dinosaur ecology. The American Naturalist 107: 391–404. Terribile LC, Diniz-Filho JAF, Rodríguez MA, Rangel TFLVB. 2009. Ecological and evolutionary components of body size: geographic variation of venomous snakes at the global scale. Biological Journal of the Linnean Society 98: 94–109. Uetz P. 2011. The reptile database. Available at: http://reptiledatabase.org [downloaded November 2011]. Vidal N, Delmas AS, David P, Cruaud C, Couloux A, Hedges SB. 2007. The phylogeny and classification of caenophidian snakes inferred from seven nuclear protein coding genes. Comptes Rendus Biologie 330: 182– 187. Vidal N, Marin J, Morini M, Donnellan S, Branch WR, Thomas R, Vences M, Wynn A, Cruaud C, Hedges SB. 2010. Blindsnake evolutionary tree reveals long history on Gondwana. Biology Letters 6: 558–561. Vitt LJ. 1987. Communities. In: Seigal RA, Collins JT, Novak SS, eds. Snakes: ecology and evolutionary biology. New York: Macmillan Publishing Company, 335–365. Wagenmakers EJ, Farrell S. 2004. AIC model selection using Akaike weights. Psychonomic Bulletin & Review 11: 192–196. Wiens JJ, Brandley MC, Reeder TW. 2006. Why does a trait evolve multiple times within a clade? Repeated evolution of snakelike body form in squamate reptiles. Evolution 60: 123–141. SUPPORTING INFORMATION Additional Supporting Information may be found in the online version of this article: Appendix S1. Appendix S2. Appendix S3. Appendix S4. Length and mass data for species. Species list and source of phylogeny, arranged by family. Phylogenetic results, model by Vidal et al. (2007). Evaluation of the allometries. © 2012 The Linnean Society of London, Biological Journal of the Linnean Society, 2013, 108, 161–172 SNAKE LENGTH–MASS ALLOMETRY 1 APPENDIX S1 LENGTH AND MASS DATA FOR SPECIES Data for length (mm) and mass (g) arranged by type of measure and by family (data for species with no exact citation are personal communication data) Snout–vent length Boidae Species SVL (mm) Mass (g) Source Boa constrictor Candoia aspera Candoia bibroni Candoia carinata Corallus caninus Corallus cookii Corallus hortulanus Epicrates cenchria Epicrates inornatus Epicrates maurus Eryx conicus Eryx jaculus Eryx johnii Eunectes murinus Lichanura trivirgata 1662 541 480 518.5 957.5 812 1021 888 1426.9 1270 240 552.5 820 2088 597 5647 293 45.2 93 586.5 210 207 830 1058.9 1767 15.9 134.5 531 5900 170.7 Zoological garden Johnson (1975) Tel Aviv University Museum (TAUM) Johnson (1975) Tiffany Doan Henderson & Winstel (1995) Tiffany Doan Vitt & Valdinger (1983), Tiffany Doan Wiley (2003) Zoological garden TAUM TAUM, zoological garden Zoological garden Rivas (2000) Rodriguez-Robles et al. (1999) Species SVL (mm) Mass (g) Source Amphiesma metusia Amphiesma sauteri Apostolepis albicollaris Apostolepis ammodites Apostolepis assimilis Apostolepis flavotorquata Atractus badius Atractus major Atractus pantostictus Atractus snethlageae Bogertophis subocularis Boiga ceylonensis Boiga irregularis Boiruna maculata Borikenophis portoricensis Chironius exoletus Chironius flavolineatus Chironius fuscus Chironius multiventris Chironius quadricarinatus Chironius scurrulus Chrysopelea ornata Clelia clelia Clelia plumbea Coluber constrictor Coluber elegantissimus Coluber flagellum Coluber sinai 663 292.7 267 232 228 460 271 403 269 282.5 1200 360 1084 1280 569.3 807 675 704.5 1695 599 1340 768 1640.7 1401 765 461.6 1160 403 134 14 4 2 4 20 8 23 15 7.75 213.4 5.9 146.9 823.5 64 147 89 69.5 650 58 750 105 2050 600 121 21.7 322 19.1 Inger et al. (1990) Inger et al. (1990) França et al. (2008) França et al. (2008) França et al. (2008) França et al. (2008) Tiffany Doan Tiffany Doan França et al. (2008) Tiffany Doan Moon & Candy (1997) TAUM Aldridge et al. (2010) Vitt & Valdinger (1983) Barun et al. (2007) França et al. (2008) França et al. (2008) Tiffany Doan Tiffany Doan França et al. (2008) Tiffany Doan Moon & Candy (1997) Tiffany Doan França et al. (2008) Ford et al. (1990) TAUM Ford et al. (1990) TAUM Colubridae 2 A. FELDMAN and S. MEIRI APPENDIX S1 Continued Species SVL (mm) Mass (g) Source Crotaphopeltis hotamboeia Dasypeltis scabra Dendrelaphis punctulatus Dendrophidion dendrophis Diadophis punctatus Dipsas catesbyi Dipsas indica Dipsas variegata Dispholidus typus Dolichophis jugularis Drepanoides anomalus Drymarchon corais Drymarchon couperi Drymobius rhombifer Drymoluber brazili Drymoluber dichrous Eirenis coronella Eirenis coronelloides Eirenis decemlineatus Eirenis modestus Eirenis rothii Elaphe carinata Elaphe climacophora Elaphe quadrivirgata 400 356.7 1006 422 459.3 397.5 308 670 856 1220.4 438.8 1249 1449 516.5 493 704.7 207.5 200 493.3 350 177 832 1155.8 860.1 13.8 15.7 166.3 20.25 19 11.3 4.5 46.7 153 692 28.6 829 1759 55.2 86 119.3 6.1 4.3 29.4 15.5 1.6 169.5 331.4 188.7 Elaphe sauromates Erythrolamprus aesculapii Erythrolamprus bizonus Gomesophis brasiliensis Helicops angulatus Helicops leopardinus Helicops modestus Hemorrhois nummifer Heterodon platirhinos Imantodes cenchoa Imantodes lentiferus Lampropeltis nigra Leptodeira annulata Leptophis ahaetulla Leptophis mexicanus Liophis almadensis Liophis lineatus Liophis maryellenae Liophis mossoroensis Liophis poecilogyrus Liophis reginae Liophis typhlus Liophis viridis Lygophis meridionalis Lygophis paucidens Lytorhynchus diadema Macroprotodon cucullatus Mastigodryas bifossatus 1363 426 716 289 306.5 240 285 854 660 685 643.8 651.7 486.5 733.7 655 285 423.5 305 449.5 371.5 438.5 549 353 444 369 350 363.3 1017 993 40 124.5 23 39.4 24 35 230 280 16.4 14.5 152.5 29.9 68.3 43.1 14 26.5 23 50.75 40.1 36 54 20.7 26 20 12.8 16.6 523 TAUM Jacobsen (1982) Shine (1991) Tiffany Doan Parker & Brown (1974) Tiffany Doan Tiffany Doan Tiffany Doan TAUM TAUM, zoological garden Tiffany Doan França et al. (2008) Stevenson et al. (2003) Tiffany Doan França et al. (2008) Tiffany Doan TAUM TAUM TAUM TAUM TAUM Moon & Candy (1997) Hasegawa & Moriguchi (1989) Hasegawa & Moriguchi (1989); Tanaka (2011); Tanaka & Ota (2002) TAUM, zoological garden França et al. (2008) Moon & Candy (1997) França et al. (2008) Tiffany Doan França et al. (2008) França et al. (2008) TAUM, zoological garden Ford et al. (1990) Tiffany Doan Tiffany Doan Faust & Blomquist (2011) Tiffany Doan Moon & Candy (1997) Moon & Candy (1997) França et al. (2008) Vitt (1983) França et al. (2008) Vitt (1983) Vitt (1983) Tiffany Doan Moon & Candy (1997), Tiffany Doan Vitt (1983) França et al. (2008) França et al. (2008) TAUM TAUM França et al. (2008) SNAKE LENGTH–MASS ALLOMETRY APPENDIX S1 Continued Species SVL (mm) Mass (g) Source Mussurana quimi Natrix maura Natrix natrix Natrix tessellata Nerodia fasciata Nerodia paucimaculata Nerodia rhombifer Nerodia sipedon 493 483 320 535.6 640 596.1 860.4 485 89 44.4 13 79.5 283 88.7 556.9 118.2 Ninia hudsoni Opheodrys aestivus Orthriophis taeniurus Oxybelis aeneus Oxyrhopus formosus Oxyrhopus guibei Oxyrhopus petolarius Oxyrhopus rhombifer Oxyrhopus trigeminus 333.5 485 755 836 575.1 439 550.8 312 455.3 14.6 17 76.2 57.5 51.2 43 29.6 21 41.3 1185 1216 700 518 504 715 771 685.5 677 454 774 440.2 476 435 305.5 695.4 283.3 780.8 530.3 261 567.6 616.5 567.5 419.5 786.1 786 919 340 246 695 62 58 47 145.3 170.5 91.5 168 50 155.4 34.4 87 22.6 26 63.6 11.5 56.6 35.9 16 62.8 134 90.6 32.5 189.3 561 89.5 10 França et al. (2008) TAUM TAUM TAUM, zoological garden Ford et al. (1990) Greene et al. (1999) Secor & Nagy (2003) King et al. (1999); Queral-Regil & King (1998); Weatherhead et al. (1995) Tiffany Doan Ford et al. (1990) Moon & Candy (1997) Vitt & Valdinger (1983) Tiffany Doan França et al. (2008) Tiffany Doan França et al. (2008) França et al. (2008); Vitt & Valdinger (1983) TAUM Zoological garden Braz et al. (2009) França et al. (2008) França et al. (2008) TAUM Vitt & Valdinger (1983) Vitt & Valdinger (1983) França et al. (2008) França et al. (2008) Moon & Candy (1997) Jacobsen (1982) França et al. (2008) TAUM Inger et al. (1990) TAUM, zoological garden TAUM TAUM TAUM França et al. (2008) Tiffany Doan Vitt & Valdinger (1983) Seigel (1992) Inger et al. (1990) Tanaka & Ota (2002) França et al. (2008) Tiffany Doan TAUM 224 372 270 449 854.6 9 13.9 12 32 211.2 Winne et al. (2005) Moon & Candy (1997) França et al. (2008) França et al. (2008) TAUM, zoological garden Pantherophis alleghaniensis Pantherophis guttatus Phalotris lativittatus Phalotris nasutus Philodryas aestivus Philodryas chamissonis Philodryas nattereri Philodryas olfersii Philodryas patagoniensis Philodryas psammophidea Philodryas viridissima Philothamnus semivariegatus Phimophis guerini Phyllorhynchus decurtatus Plagiopholis tyani Platyceps collaris Platyceps florulentus Platyceps rhodorachis Platyceps rogersi Pseudablabes agassizii Pseudoboa coronate Pseudoboa nigra Regina grahami Rhabdophis nuchalis Rhabdophis tigrinus Rhachidelus brazili Rhinobothryum lentiginosum Rhynchocalamus melanocephalus Seminatrix pygaea Sibon nebulatus Sibynomorphus mikanii Simophis rhinostoma Spalerosophis diadema 3 4 A. FELDMAN and S. MEIRI APPENDIX S1 Continued Species SVL (mm) Mass (g) Source Spilotes pullatus Stegonotus cucullatus Storeria dekayi Storeria occipitomaculata Tachymenis peruviana Tantilla coronata 1404 1040 229.8 230.5 346 188.5 552.5 298 4.7 7.3 29.2 2.5 Tantilla gracilis Tantilla melanocephala Telescopus dhara Telescopus fallax Telescopus hoogstraali Telescopus semiannulatus Thamnodynastes hypoconia 133.8 228 731 488 658.3 400 317.7 1.6 7 82 33.2 74.6 9.5 20.4 Thamnodynastes pallidus Thamnodynastes rutilus Thamnophis butleri Thamnophis couchii Thamnophis marcianus Thamnophis proximus Thamnophis radix Thamnophis scalaris Thamnophis sirtalis 585 275 381 510.6 521 635.3 435.6 395 549.4 53 21 24.8 61 90 58.9 50.9 42 104.2 Thelotornis capensis Thelotornis kirtlandii Trimorphodon biscutatus Tropidonophis mairii Virginia striatula Virginia valeriae Xenochrophis flavipunctatus Xenochrophis piscator Xenodon merremi Xenodon nattereri Xenopholis scalaris Xenopholis undulatus Xenoxybelis argenteus Zamenis longissimus 701.5 877 823 644.5 183 232.7 667.5 555 615.3 245 254.9 268 660 645 78.8 69 84.3 119.1 3.3 7.2 250.7 131.6 201.8 14 8.8 10 25 199 Vitt & Valdinger (1983) Shine (1991) Ford et al. (1990) Ford et al. (1990) Tiffany Doan Semlitsch et al. (1981); Todd et al. (2008) Cobb (2004) França et al. (2008) TAUM TAUM TAUM TAUM França et al. (2008); Vitt & Valdinger (1983) Vitt & Valdinger (1983) França et al. (2008) Ford & Killebrew (1983) Lind & Welsh (1990) Ford & Karges (1987) Lancaster & Ford (2003) King et al. (1999) Manjarrez et al. (2007) King et al. (1999); Moon & Candy (1997) Shine et al. (1996b) TAUM Moon & Candy (1997) Shine (1991); Brown & Shine (2002) Ford et al. (1990) Ford et al. (1990) Karns et al. (2010) Brooks et al. (2009) França et al. (2008); Vitt (1983) França et al. (2008) Tiffany Doan França et al. (2008) Tiffany Doan TAUM Elapidae Species SVL (mm) Mass (g) Source Aspidelaps scutatus Bungarus ceylonicus Bungarus flaviceps Demansia vestigiata Dendroaspis angusticeps Dendroaspis polylepis Enhydrina schistosa Hoplocephalus bungaroides Hoplocephalus stephensii Lapemis curtus 391.5 770 825 610.7 1245 1410 792.9 685 621 508.6 75.7 47.7 90.3 60.8 282.4 651.7 314.23 71 84 147.1 Shine et al. (1996a) TAUM Moon & Candy (1997) Fearn & Trembath (2009) Moon & Candy (1997) Jacobsen (1982) Lobo et al. (2004) Shine & Fitzgerald (1989) Fitzgerald et al. (2004) Lobo et al. (2004) SNAKE LENGTH–MASS ALLOMETRY 5 APPENDIX S1 Continued Species SVL (mm) Mass (g) Source Laticauda colubrina Micrurus frontalis Micrurus ibiboboca Micrurus lemniscatus Naja annulifera Naja atra Naja mossambica Naja naja Naja nigricollis Notechis scutatus Simoselaps bertholdi Simoselaps bimaculatus Simoselaps calonotus Simoselaps fasciolatus Simoselaps semifasciatus Walterinnesia aegyptia 921 650 643.5 679 858.1 1000.7 586.2 1570 1010 1258 203.4 306.5 214.8 241.4 261.2 810 355 80 48.5 38.5 391.2 311.1 171.3 1497.5 256.7 835.5 7.6 8.9 5 9.3 12.7 321 Moon & Candy (1997) França et al. (2008) Vitt & Valdinger (1983) Tiffany Doan Jacobsen (1982) Ji & Wang (2005) Jacobsen (1982) TAUM TAUM Schwaner & Sarre (1988) How & Shine (1999) How & Shine (1999) How & Shine (1999) How & Shine (1999) How & Shine (1999) TAUM Homalopsidae Species SVL (mm) Mass (g) Source Cerberus rynchops Enhydris bocourti Enhydris enhydris Enhydris jagorii Enhydris longicauda Enhydris plumbea Enhydris subtaeniata Erpeton tentaculatum Fordonia leucobalia Gerarda prevostiana Homalopsis buccata 460 552.5 490.5 484 465.5 389 537.5 480 461 365 729.7 60.4 232.4 89.6 245 134.2 59.6 232.2 92.9 68.2 23.2 296.7 Karns et al. (2002) Brooks et al. (2009) Brooks et al. (2009) Karns et al. (2010) Brooks et al. (2009) Karns et al. (2005) Karns et al. (2010) Brooks et al. (2009) Karns et al. (2002) Karns et al. (2002) Brooks et al. (2009); Karns et al. (2010) Lamprophiidae Species SVL (mm) Mass (g) Source Amblyodipsas polylepis Amblyodipsas ventrimaculata Aparallactus capensis Atractaspis bibronii Atractaspis engaddensis Atractaspis irregularis Atractaspis microlepidota Boaedon fuliginosus Gonionotophis capensis Gonionotophis nyassae Homoroselaps lacteus Lycodonomorphus rufulus Lycophidion capense Macrelaps microlepidotus Malpolon monspessulanus Micrelaps muelleri 528.5 295.5 256.3 467.5 616.5 405 677 466.5 627 382.94 465 405 283.5 650 1166 359.3 81.5 7.4 4.9 34.3 57.5 21.2 93.5 30.8 158.9 19.8 21.3 33.1 10.8 176.6 1257 7.7 Shine et al. (2006b) Shine et al. (2006b) Jacobsen (1982) Shine et al. (2006b) TAUM TAUM TAUM Jacobsen (1982), TAUM Jacobsen (1982) Jacobsen (1982) TAUM TAUM Jacobsen (1982) Shine et al. (2006b) TAUM, zoological garden TAUM 6 A. FELDMAN and S. MEIRI APPENDIX S1 Continued Species SVL (mm) Mass (g) Source Micrelaps tchernovi Pararhadinaea melanogaster Prosymna sundevallii Psammophis aegyptius Psammophis brevirostris Psammophis jallae Psammophis leopardinus Psammophis mossambicus Psammophis namibensis Psammophis notostictus Psammophis schokari Psammophis subtaeniatus Psammophis trigrammus Psammophis trinasalis Psammophylax tritaeniatus Pseudaspis cana Rhagerhis moilensis Xenocalamus bicolor Xenocalamus mechowii 315 215.6 219.7 720 648.5 614 703.5 787 579 488.5 623 598 647.5 511.5 405 950 250.2 476 588 3 2.9 7.2 197.9 119.05 62.05 145.15 213.95 50.35 29.8 87 68.15 51.25 41.6 29.3 492.9 8.07 27 23.5 TAUM Labanowski & Lowin (2011) Jacobsen (1982) TAUM Shine et al. (2006a) Shine et al. (2006a) Shine et al. (2006a) Shine et al. (2006a) Shine et al. (2006a) Shine et al. (2006a) TAUM, zoological garden Shine et al. (2006a) Shine et al. (2006a) Shine et al. (2006a) Shine et al. (2006a) TAUM TAUM Shine et al. (2006b) Shine et al. (2006b) Pythonidae Species SVL (mm) Mass (g) Source Antaresia maculosa Broghammerus reticulates Morelia spilota Morelia viridis Python regius Python sebae 1154 3061.3 1587.6 1021 1125 3370 530 11769.3 2120.15 563.2 1324 13250 Trembath (2008) Shine et al. (1998) Pearson et al. (2002) TAUM Aubret et al. (2005), zoological garden TAUM Scolecophidia Species SVL (mm) Mass (g) Source Epictia albipuncta Epictia tenella Leptotyphlops distanti Liotyphlops ternetzii Myriopholis macrorhyncha Ramphotyphlops bicolor Ramphotyphlops braminus Rhinotyphlops simonii Siagonodon septemstriatus Tetracheilostoma breuili Tetracheilostoma carlae Trilepida fuliginosa Trilepida macrolepis Typhlops minuisquamus Typhlops reticulatus Typhlops vermicularis 144.5 165.7 143.5 207 232.1 234 143.4 198.2 160 99.9 93.7 207.25 279.7 264 248.3 187.9 1.4 1.2 0.57 3 1.38 10.39 0.85 0.95 1.5 0.61 0.6 4.6 9.5 16.5 22 2.2 Marinus Hoogmoed Alessandro Costa Menks Jacobsen (1982) França et al. (2008), Marinus Hoogmoed TAUM Dale Nimmo Kamosawa & Ota (1996) TAUM Marinus Hoogmoed Hedges (2008) Hedges (2008) França et al. (2008), Marinus Hoogmoed Alessandro Costa Menks Marinus Hoogmoed Alessandro Costa Menks, Marinus Hoogmoed TAUM SNAKE LENGTH–MASS ALLOMETRY 7 APPENDIX S1 Continued Viperidae Species SVL (mm) Mass (g) Source Agkistrodon bilineatus Agkistrodon contortrix Agkistrodon piscivorus Atheris squamigera Bitis arietans Bitis caudalis Bitis cornuta Bitis gabonica Bitis peringueyi Bitis schneideri Bothriopsis bilineata Bothropoides neuwiedi Bothrops moojeni Causus defilippii Causus resimus Causus rhombeatus Cerastes cerastes Cerastes gasperettii Cerastes vipera Crotalus cerastes Crotalus durissus Crotalus horridus Crotalus molossus Crotalus oreganus Crotalus polystictus Crotalus pricei Crotalus ravus Crotalus triseriatus Crotalus viridis 337 610.5 580 477.5 839.5 302.5 325 331 230 198.7 422 413 618 317 531.7 630 530.1 547 214.6 428.8 1344.5 946 885 920 607 421.4 683 180 678.7 51.1 217.2 204 44.5 743.3 24.1 32.6 47.5 8.6 15.8 185 43 217 21.6 53.1 94.8 186.6 144 9.9 94.7 2440.5 581.9 438.4 502.6 188.9 62.6 223 4.4 221.1 Daboia palaestinae Daboia russelii Echis carinatus Echis coloratus Echis pyramidum Eristicophis macmahoni Gloydius blomhoffii Gloydius himalayanus Gloydius shedaoensis Macrovipera deserti Macrovipera lebetina Macrovipera mauritanica Montivipera bornmuelleri Montivipera latifii Montivipera raddei Montivipera xanthina Ovophis zayuensis Protobothrops jerdonii Pseudocerastes fieldi Pseudocerastes persicus Rhinocerophis alternatus Rhinocerophis itapetiningae Sistrurus catenatus 872 930 550.5 622.7 457.8 542 410 481 634 690 768 1080 654 578 848.7 942 435 633.5 612.5 625 760 304 535 437.5 675 178.4 147.7 76.6 125 63.7 75.7 124 244.2 380 855 193 104 317.3 351.3 72 166.5 185.2 215.3 229.6 38 167.2 Moon & Candy (1997) Ford et al. (1990); Schuett & Gillingham (1989) Ford et al. (1990) TAUM TAUM TAUM TAUM TAUM TAUM Maritz & Alexander (2011) Tiffany Doan França et al. (2008) França et al. (2008) TAUM TAUM TAUM TAUM TAUM TAUM TAUM Vitt & Valdinger (1983) Brown (1991); Moon & Candy (1997) Moon & Candy (1997) TAUM Setser et al. (2010); Moon & Candy (1997) Prival et al. (2002) Moon & Candy (1997) Manjarrez et al. (2007) Ashton & Patton (2001); Diller & Wallace (2002), Moon & Candy (1997) TAUM TAUM Moon & Candy (1997), TAUM Moon & Candy (1997), TAUM TAUM TAUM TAUM TAUM Li-Xin et al. (2002) TAUM TAUM TAUM TAUM TAUM Moon & Candy (1997), TAUM TAUM Inger et al. (1990) Inger et al. (1990) TAUM TAUM TAUM França et al. (2008) Moon & Candy (1997) 8 A. FELDMAN and S. MEIRI APPENDIX S1 Continued Species SVL (mm) Mass (g) Source Sistrurus miliarius Trimeresurus gracilis Trimeresurus kanburiensis Trimeresurus stejnegeri Vipera ammodytes Vipera aspis Vipera berus Vipera kaznakovi 369 296 825 707 611 517 330.1 493.5 40.6 34.5 283.5 175.5 210.9 74.1 32.3 52.1 Ford et al. (1990) Chia-Fan & Ming-Chung (2008) Moon & Candy (1997) Moon & Candy (1997) TAUM Bonnet et al. (2000), TAUM Forsman & Lindell (1996), TAUM TAUM Total length Boidae Species TL (mm) Mass (g) Source Boa constrictor Calabaria reinhardtii Candoia bibroni Candoia carinata Charina bottae Corallus caninus Corallus hortulanus Epicrates cenchria Epicrates maurus Eryx conicus Eryx jaculus Eryx johnii Eunectes murinus 1848.3 837 560 780 488.65 1142.5 1273.8 1023.3 1440 260 599.3 900 2426 5646.7 183 45.2 530 39.8 586.5 207.2 829.6 1767 15.9 134.5 531 5900 Zoolological garden Angelici et al. (2000) TAUM Seymor (1987) Hoyer & Stewart (2000), Peabody et al. (1975) Tiffany Doan Tiffany Doan Vitt & Valdinger (1983), Tiffany Doan Zoological garden TAUM TAUM Zoological garden Rivas (2000) Colubridae Species TL (mm) Mass (g) Source Ahaetulla prasina Amphiesma metusia Amphiesma sauteri Apostolepis albicollaris Apostolepis ammodites Apostolepis assimilis Apostolepis flavotorquata Arizona elegans Atractus badius Atractus major Atractus pantostictus Atractus snethlageae Boiga ceylonensis Boiga cynodon Boiga dendrophila Boiga irregularis Boiruna maculata Borikenophis portoricensis Chironius exoletus Chironius flavolineatus Chironius fuscus Chironius multiventris 1380 885 414.25 297 259 251 502 1070 306.8 456 300 321 460 2350 1260 1320 1517 816.9 1276 1116 1080 2496 78.2 134 14 4 2 4 20 161 8.3 23 15 7.75 5.9 417.3 182 402 823.5 64 147 89 70 650 Seymor (1987) Inger et al. (1990) Inger et al. (1990) França et al. (2008) França et al. (2008) França et al. (2008) França et al. (2008) Seymor (1987) Tiffany Doan Tiffany Doan França et al. (2008) Tiffany Doan TAUM Quinn & Neitman (1978) Seymor (1987) Seymor (1987) Vitt & Valdinger (1983) Barun et al. (2007) França et al. (2008) França et al. (2008) Tiffany Doan Tiffany Doan SNAKE LENGTH–MASS ALLOMETRY 9 APPENDIX S1 Continued Species TL (mm) Mass (g) Source Chironius quadricarinatus Chironius scurrulus Chrysopelea ornata Clelia clelia Clelia plumbea Coelognathus radiatus Coluber constrictor Coluber elegantissimus Coluber flagellum Coluber sinai Coronella austriaca 959 1720 1050 2040.7 1716 740 1160 604.4 1810 543.7 495 58 750 145 2050 600 50.5 182 21.7 475 19.1 50 Coronella girondica Crotaphopeltis hotamboeia Dendrelaphis calligastra Dendrelaphis caudolineatus Dendrelaphis punctulatus Dendrophidion dendrophis Diadophis punctatus Dinodon rufozonatum Dipsas catesbyi Dipsas indica Dipsas variegata Dispholidus typus Dolichophis jugularis Drepanoides anomalus Drymarchon corais Drymarchon couperi Drymobius rhombifer Drymoluber brazili Drymoluber dichrous Eirenis coronella Eirenis coronelloides Eirenis decemlineatus Eirenis modestus Eirenis rothii Elaphe quatuorlineata Elaphe sauromates Erythrolamprus aesculapii Gomesophis brasiliensis Helicops angulatus Helicops leopardinus Helicops modestus Hemorrhois hippocrepis Hemorrhois nummifer Hierophis viridiflavus Imantodes cenchoa Imantodes lentiferus Lampropeltis getula Lampropeltis nigra Leptodeira annulata Leptophis ahaetulla Liophis almadensis Liophis lineatus 650 448 1030 937.5 1185 742 535.1 780 532.2 412 921.5 1156 1698.3 569.2 1553 1725.5 701.5 622 979.7 261.5 254 666.7 454.2 219 1600 1612.3 485 359 426.2 360 378 1100 1073 1153.3 954.2 913.6 1120 745.3 654.3 1144.7 375 550.5 55 13.8 83.7 54 130.25 20.25 17.2 160.9 11.3 4.5 46.7 153 691.9 28.6 829 1759 55.2 86 119.3 6.1 4.3 29.4 15.5 1.6 400 993 40 23 39.4 24 35 220 230 172.3 16.4 14.5 258 152.5 29.9 68.3 14 26.5 França et al. (2008) Tiffany Doan Seymor (1987) Tiffany Doan França et al. (2008) Seymor (1987) Seymor (1987) TAUM Seymor (1987) TAUM Luiselli, Capula & Shine (1996); Zuffi et al. (2010) Zuffi et al. (2010) TAUM Seymor (1987) Seymor (1987) Seymor (1987) Tiffany Doan Parker & Brown (1974); Seymor (1987) Dieckmann et al. (2010) Tiffany Doan Tiffany Doan Tiffany Doan TAUM TAUM, zoological garden Tiffany Doan França et al. (2008) Stevenson et al. (2003) Tiffany Doan França et al. (2008) Tiffany Doan TAUM TAUM TAUM TAUM TAUM TAUM TAUM, zoological garden França et al. (2008) França et al. (2008) Tiffany Doan França et al. (2008) França et al. (2008) Zuffi et al. (2010) TAUM, zoological garden Capula et al. (1995); Zuffi et al. (2010) Tiffany Doan Tiffany Doan Seymor (1987) Faust & Blomquist (2011) Tiffany Doan Vitt & Valdinger (1983), Tiffany Doan França et al. (2008) Vitt (1983) 10 A. FELDMAN and S. MEIRI APPENDIX S1 Continued Species TL (mm) Mass (g) Source Liophis maryellenae Liophis mossoroensis Liophis poecilogyrus Liophis reginae Liophis typhlus Liophis viridis Lygophis meridionalis Lygophis paucidens Lytorhynchus diadema Macroprotodon cucullatus Mastigodryas bifossatus Mussurana quimi Natrix maura Natrix natrix Natrix tessellata Nerodia sipedon 399 537.35 453 570.8 720 456 611 496 412 433.7 1410 622 615 400 677.4 786.2 23 50.75 40.1 35.9 47 20.75 26 20 12.8 16.6 523 89 44.4 13 79.5 174 Nerodia taxispilota Ninia hudsoni Oxybelis aeneus Oxyrhopus formosus Oxyrhopus guibei Oxyrhopus petolarius Oxyrhopus rhombifer Oxyrhopus trigeminus 798 389.5 1332 741 556 809.8 389 563.3 228 14.65 57.5 51.25 43 34 21 41.3 Pantherophis alleghaniensis Pantherophis guttatus Pantherophis obsoletus Pantherophis spiloides Phalotris lativittatus Phalotris nasutus Philodryas aestivus Philodryas chamissonis Philodryas nattereri Philodryas olfersii Philodryas patagoniensis Philodryas psammophidea Phimophis guerini Phyllorhynchus decurtatus Pituophis melanoleucus Plagiopholis styani Platyceps collaris Platyceps florulentus Platyceps rhodorachis Platyceps rogersi Pseudablabes agassizii Pseudoboa coronata Pseudoboa nigra Ptyas korros Rhabdophis nuchalis Rhachidelus brazili Rhinechis scalaris Rhinobothryum lentiginosum 1467 1453.3 1380 1348 764 576 718 955 1066.5 947.5 929 610 607 510 1620 347.5 985.3 377.7 1099.3 726.5 346 743.8 809.5 930 517.8 999 1500 1155 246 695.3 510 322.8 62 58 47 145.3 170.5 91.5 168 50 87 22.6 747 26 63.6 11.5 56.6 35.9 16 62.8 134 201 32.5 561 500 89.5 França et al. (2008) Vitt (1983) Vitt (1983) Tiffany Doan Tiffany Doan Vitt (1983) França et al. (2008) França et al. (2008) TAUM TAUM França et al. (2008) França et al. (2008) TAUM TAUM TAUM, zoological garden King et al. (1999); Weatherhead et al. (1995) Seymor (1987) Tiffany Doan Vitt & Valdinger (1983) Tiffany Doan França et al. (2008) Tiffany Doan França et al. (2008) Vitt & Valdinger (1983); França et al. (2008) TAUM Zoological garden Seymor (1987) Schumacher et al. (1997) Braz et al. (2009) França et al. (2008) França et al. (2008) TAUM Vitt & Valdinger (1983) Vitt & Valdinger (1983) França et al. (2008) França et al. (2008) França et al. (2008) TAUM Seymor (1987) Inger et al. (1990) TAUM, zoological garden TAUM TAUM TAUM França et al. (2008) Tiffany Doan Vitt & Valdinger (1983) Seymor (1987) Inger et al. (1990) França et al. (2008) Zuffi et al. (2010) Tiffany Doan SNAKE LENGTH–MASS ALLOMETRY 11 APPENDIX S1 Continued Species Rhynchocalamus melanocephalus Sibynomorphus mikanii Simophis rhinostoma Spalerosophis diadema Spilotes pullatus Tachymenis peruviana Tantilla coronata TL (mm) Mass (g) 420 10 332 565 1042.8 1803.5 427.5 231.8 12 32 211.2 552.5 29.2 2.5 Tantilla melanocephala Telescopus dhara Telescopus fallax Telescopus hoogstraali Telescopus semiannulatus Thamnodynastes hypoconia 301 853 567.95 779.8 490 407 7 81.96 33.2 74.6 9.5 20.4 Thamnodynastes pallidus Thamnodynastes rutilus Thamnophis radix Thamnophis sirtalis Thelotornis capensis Thelotornis kirtlandii Xenochrophis flavipunctatus Xenodon merremi Xenodon nattereri Xenopholis scalaris Xenopholis undulatus Xenoxybelis argenteus Zamenis lineatus Zamenis longissimus 800 384 552.7 630.85 1109.5 1369 898.56 718.3 289 302.6 316 1105 930 795 53 21 50.9 73.03 78.8 69 250.7 201.7 14 8.8 10 25 190 199.3 Source TAUM França et al. (2008) França et al. (2008) TAUM, zoological garden Vitt & Valdinger (1983) Tiffany Doan Semlitsch et al. (1981); Todd et al. (2008) França et al. (2008) TAUM TAUM TAUM TAUM Vitt & Valdinger (1983); França et al. (2008) Vitt & Valdinger (1983) França et al. (2008) King et al. (1999) King et al. (1999) Shine et al. (1996b) TAUM Karns et al. (2010) Vitt (1983); França et al. (2008) França et al. (2008) Tiffany Doan França et al. (2008) Tiffany Doan Zuffi et al. (2010) TAUM Elapidae Species TL (mm) Mass (g) Source Acanthophis antarcticus Acanthophis pyrrhus Aspidelaps scutatus Austrelaps superbus Bungarus ceylonicus Cacophis squamulosus Chitulia belcheri Chitulia inornata Chitulia ornata Cryptophis nigrescens Demansia vestigiata Disteira major Disteira stokesii Enhydrina schistosa Furina diadema Furina tristis Hemiaspis signata Hoplocephalus stephensii 567 585 448.7 553 850 726 852 645 614 481 874.3 910 470 892.8 388 860 529 878 252 148 75.75 339 47.7 45.7 260 156 107 35 89.7 258 52 314.2 15 147 35.8 104 Seymor (1987) Seymor (1987) Shine et al. (1996a) Seymor (1987) TAUM Seymor (1987) Seymor (1987) Seymor (1987) Seymor (1987) Seymor (1987) Fearn & Trembath (2009) Seymor (1987) Seymor (1987) Lobo et al. (2004) Seymor (1987) Seymor (1987) Seymor (1987) Seymor (1987) 12 A. FELDMAN and S. MEIRI APPENDIX S1 Continued Species TL (mm) Mass (g) Source Hydrelaps darwiniensis Lapemis curtus Lapemis hardwickii Laticauda laticaudata Leioselasma cyanocincta Leioselasma elegans Micrurus frontalis Micrurus ibiboboca Micrurus lemniscatus Micrurus tener Naja atra Naja naja Naja nigricollis Notechis scutatus Oxyuranus microlepidotus Oxyuranus scutellatus Pelamis platura Pseudechis australis Pseudechis porphyriacus Pseudolaticauda semifasciata Pseudonaja affinis Pseudonaja nuchalis Pseudonaja textilis Rhinoplocephalus bicolor Simoselaps fasciolatus Simoselaps incinctus Simoselaps littoralis Suta flagellum Tropidechis carinatus Vermicella annulata Walterinnesia aegyptia 391 566.5 635 883 568 1980 692 681.5 734 610 1166.3 1843.5 1250 870 1660 22.4 147.1 330 165 39.3 2450 80 48.5 38.5 33.8 311.1 1497.5 256.7 226 838 Seymor (1987) Lobo et al. (2004) Seymor (1987) Seymor (1987) Seymor (1987) Seymor (1987) França et al. (2008) Vitt & Valdinger (1983) Tiffany Doan Campbell (1973) Ji & Wang (2005) TAUM TAUM Seymor (1987) Seymor (1987) 2100 637 1440 766 1020 753 30 642 304 641 Seymor Seymor Seymor Seymor Seymor (1987) (1987) (1987) (1987) (1987) 1070 1380 1360 340 291 278 215 256 821 482 927.3 368 464 469 17.4 9.5 11.5 7.75 7.05 170 14.5 320.6 Seymor Seymor Seymor Seymor Seymor Seymor Seymor Seymor Seymor Seymor TAUM (1987) (1987) (1987) (1987) (1987) (1987) (1987) (1987) (1987) (1987) Homalopsidae Species TL (mm) Mass (g) Source Cerberus australis Cerberus rynchops Enhydris jagorii Enhydris polylepis Enhydris subtaeniata Fordonia leucobalia Gerarda prevostiana Homalopsis buccata 500 500 621.6 664 645 528 420 953.9 36.8 36.5 245 132 232.2 96.8 23.2 347.2 Seymor (1987) Karns et al. (2002) Karns et al. (2010) Seymor (1987) Karns et al. (2010) Karns et al. (2002); Seymor (1987) Karns et al. (2002) Karns et al. (2010) SNAKE LENGTH–MASS ALLOMETRY 13 APPENDIX S1 Continued Lamprophiidae Species TL (mm) Mass (g) Source Amblyodipsas polylepis Amblyodipsas ventrimaculata Atractaspis aterrima Atractaspis bibronii Atractaspis engaddensis Atractaspis irregularis Atractaspis microlepidota Boaedon fuliginosus Homoroselaps lacteus Lycodonomorphus rufulus Macrelaps microlepidotus Malpolon monspessulanus Micrelaps muelleri Micrelaps tchernovi Pararhadinaea melanogaster Psammophis aegyptius Psammophis brevirostris Psammophis jallae Psammophis leopardinus Psammophis mossambicus Psammophis namibensis Psammophis notostictus Psammophis schokari Psammophis subtaeniatus Psammophis trigrammus Psammophis trinasalis Psammophylax tritaeniatus Pseudaspis cana Rhagerhis moilensis Xenocalamus bicolor Xenocalamus mechowii 571.5 322 522 484.5 664.2 434 743 725 520 565 771.5 1484 383 340 251.7 1005 964.5 914.5 1016.5 1109.5 830.5 706.5 938.4 924 1018 748 515 1150 310.2 518.5 631 81.5 7.4 27.5 34.3 57.5 21.2 93.5 50.9 21.3 33.1 176.6 1257 7.7 3 2.9 197.9 119.05 62.05 145.15 213.95 50.35 29.8 86.6 68.15 51.25 41.6 29.3 492.9 8.07 27 23.5 Shine et al. (2006b) Shine et al. (2006b) Gower et al. (2004) Shine et al. (2006b) TAUM TAUM TAUM TAUM TAUM TAUM Shine et al. (2006b) TAUM, zoological garden TAUM TAUM Labanowski & Lowin (2011) TAUM Shine et al. (2006a) Shine et al. (2006a) Shine et al. (2006a) Shine et al. (2006a) Shine et al. (2006a) Shine et al. (2006a) TAUM, zoological garden Shine et al. (2006a) Shine et al. (2006a) Shine et al. (2006a) Shine et al. (2006a) TAUM TAUM Shine et al. (2006b) Shine et al. (2006b) Pythonidae Species TL (mm) Mass (g) Source Antaresia maculosa Antaresia perthensis Aspidites melanocephalus Aspidites ramsayi Broghammerus reticulatus Liasis fuscus Liasis olivaceus Morelia spilota Morelia viridis Python bivittatus Python regius 1259 769 1310 1950 3522.52 1490 2400 1683.8 1210 3220 1216 530 339 1362 3900 11769.35 953 3305 1924.4 563.2 21750 1378 Python sebae 3735 13250 Trembath (2008) Seymor (1987) Seymor (1987) Seymor (1987) Shine et al. (1998) Seymor (1987) Seymor (1987) Pearson et al. (2002); Seymor (1987) TAUM Van Mierop & Barnard (1976) Gorzula et al. (1997); Aubret et al. (2005), zoological garden TAUM 14 A. FELDMAN and S. MEIRI APPENDIX S1 Continued Scolecophidia Species TL (mm) Mass (g) Source Austrotyphlops australis Austrotyphlops bituberculatus Epictia albipuncta Epictia tenella Liotyphlops ternetzii Myriopholis macrorhyncha Ramphotyphlops bicolor Ramphotyphlops polygrammicus Rhinotyphlops simonii Siagonodon septemstriatus Tetracheilostoma breuili Tetracheilostoma carlae Trilepida fuliginosa Trilepida macrolepis Typhlops minuisquamus Typhlops reticulatus Typhlops tasymicris Typhlops vermicularis 328 247.6 156 177.7 211 254.9 241.3 510 201.1 165 106.8 99.4 226.85 305.5 272.6 255.9 227 191.5 48 3.4 1.4 1.2 3 1.4 10.4 43.3 0.95 1.5 0.61 0.6 4.6 9.5 16.5 22 1.9 2.2 Seymor (1987) Dale Nimmo Marinus Hoogmoed Alessandro Costa Menks França et al. (2008), Marinus Hoogmoed TAUM Dale Nimmo Seymor (1987) TAUM Marinus Hoogmoed Hedges (2008) Hedges (2008) França et al. (2008), Marinus Hoogmoed Alessandro Costa Menks Marinus Hoogmoed Alessandro Costa Menks Bentz et al. (2011) TAUM Viperidae Species TL (mm) Mass (g) Source Agkistrodon piscivorus Atheris squamigera Bitis arietans Bitis caudalis Bitis cornuta Bitis gabonica Bitis peringueyi Bothriechis schlegelii Bothriopsis bilineata Bothropoides neuwiedi Bothrops moojeni Causus defilippii Causus resimus Causus rhombeatus Cerastes cerastes Cerastes gasperettii Cerastes vipera Crotalus atrox Crotalus cerastes Crotalus durissus Crotalus oreganus Crotalus ruber Crotalus triseriatus Crotalus viridis Daboia palaestinae Daboia russelii Echis carinatus Echis coloratus Echis pyramidum 1060 570 927 327.75 360 1053 242.5 650 468 473 719 338 576 685 590.2 603.9 239.4 1230 496.5 1461.5 1044.5 1060 222 850 984 1070 516.25 658.6 510.3 861 44.5 743.3 24.1 32.65 4023.7 8.6 102 185 43 217 21.6 53.13 94.8 186.6 144.3 9.9 1000 114.87 2440.5 502.6 285 4.41 415 437.6 675 113.7 92.8 76.6 Seymor (1987) TAUM TAUM TAUM TAUM Broadley et al. (2003), TAUM TAUM Seymor (1987) Tiffany Doan França et al. (2008) França et al. (2008) TAUM TAUM TAUM TAUM TAUM TAUM Seymor (1987) Seymor (1987), TAUM Vitt & Valdinger (1983) TAUM Seymor (1987) Manjarrez et al. (2007) Seymor (1987) TAUM TAUM Seymor (1987)TAUM TAUM TAUM SNAKE LENGTH–MASS ALLOMETRY 15 APPENDIX S1 Continued Species Eristicophis macmahoni Gloydius blomhoffii Gloydius himalayanus Macrovipera deserti Macrovipera lebetina Macrovipera mauritanica Montivipera bornmuelleri Montivipera latifii Montivipera raddei Montivipera xanthina Ovophis zayuensis Protobothrops jerdonii Pseudocerastes fieldi Pseudocerastes persicus Rhinocerophis alternatus Rhinocerophis itapetiningae Vipera ammodytes Vipera aspis Vipera Vipera Vipera Vipera berus kaznakovi latastei ursinii TL (mm) Mass (g) Source 125 TAUM 482 511 800 871.2 1260 63.7 75.7 244.2 380 855 TAUM TAUM TAUM TAUM TAUM 705.3 192.7 TAUM 621.5 853.75 1027.5 530.5 752.7 687.5 715 103.9 255.5 351.3 72 166.5 185.2 215.3 TAUM Zuffi et al. (2010), TAUM TAUM Inger et al. (1990) Inger et al. (1990) TAUM TAUM 900 229.6 TAUM 343 38 682 605 210.9 85.2 595 567.7 551 550 450 REFERENCES FOR THE LENGTH AND MASS DATA Aldridge RD, Siegel DS, Bufalino AP, Wisniewski SS, Jellen BC. 2010. A multiyear comparison of the male reproductive biology of the brown treesnake (Boiga irregularis) from Guam. Australian Journal of Zoology 58: 24– 32. Andren C. 1982. Effect of prey density on reproduction, foraging and other activities in the adder, Vipera berus. Amphibia-Reptilia 3: 81–96. Angelici FM, Inyang MA, Effah C, Luiselli L. 2000. Analysis of activity patterns and habitat use of radiotracked African burrowing pythons Calabaria reinhardtii. Israel Journal of Zoology 46: 131–141. Ashton KG, Patton TM. 2001. Movement and reproductive biology of female midget faded rattlesnakes, Crotalus viridis concolor, in Wyoming. Copeia 2001: 229–234. Aubret F, Bonnet X, Harris M, Maumelat S. 2005. Sex differences in body size and ectoparasite load in the ball python, Python regius. Journal of Herpetology 39: 315– 320. 74.5 52.1 87 65 França et al. (2008) TAUM Naulleau & Girons (1981); Zuffi et al. (2010), TAUM Andren (1982), TAUM TAUM Zuffi et al. (2010) Zuffi et al. (2010) Barun A, Perry G, Henderson RW, Powel R. 2007. Alsophis portoricensis anegadae (Squamata: Colubridae): morphometric characteristics, activity patterns, and habitat use. Copeia 2007: 93–100. Bentz EJ, Rodriguez MJR, John R, Henderson RW, Powell R. 2011. Population densities, activity, microhabitats, and thermal biology of a unique crevice- and litterdwelling assemblage of reptiles on Union Island, St. Vincent and the Grenadines. Herpetological Conservation and Biology 6: 40–50. Bonnet X, Naulleau G, Shine R, Lourdais O. 2000. Reproductive versus ecological advantages to larger body size in female snakes, Vipera aspis. Oikos 89: 509–518. Braz HBP, Araujo CO, Almeida-Santos SM. 2009. Life history traits of the snake Phalotris lativittatus (Xenodontinae: Elapomorphini) from the Brazilian Cerrado. Herpetology Notes 2: 163–164. Broadley DG, Doria CT, Wigge J. 2003. Snakes of Zambia: an Atlas and field guide. Frankfurt am Main: Edition Chimaira. Brooks SE, Allison EH, Gill JA, Reynolds JD. 2009. Reproductive and trophic ecology of an assemblage of 16 A. FELDMAN and S. MEIRI aquatic and semi-aquatic snakes in Tonle Sap, Cambodia. Copeia 2009: 7–20. Brown GP, Shine R. 2002. Reproductive ecology of a tropical natricine snake, Tropidonophis mairii (Colubridae). Journal of Zoology 258: 63–72. Brown WS. 1991. Female reproductive ecology in a northern population of the timber rattlesnake, Crotalus horridus. Herpetologica 47: 101–115. Campbell JA. 1973. A captive hatching of Micrurus fulvius tenere (Serpentes, Elapidae). Journal of Herpetology 7: 312– 315. Capula M, Filippi E, Luiselli L. 1995. Annual mating in female Colubrid snakes with irregular reproductive frequency. Herpetozoa 8: 11–15. Chia-Fan L, Ming-Chung T. 2008. Food habits of the Taiwanese mountain pitviper, Trimeresurus gracilis. Zoological Studies 47: 697–703. Cobb VA. 2004. Diet and prey size of the flathead snake, Tantilla gracilis. Copeia 2004: 397–402. Dieckmann S, Norval G, Mao JJ. 2010. A description of an Asian king snake (Dinodon rufozonatum rufozonatum [Cantor, 1842]) clutch size from central western Taiwan. Herpetology Notes 3: 313–314. Diller LV, Wallace RL. 2002. Growth, reproduction, and survival in a population of Crotalus viridis oreganus in north central Idaho. Herpetological Monographs 16: 26–45. Faust TM, Blomquist SM. 2011. Size and growth in two populations of black kingsnakes, Lampropeltis nigra, in east Tennessee. Southern Eastern Naturalist 10: 40–422. Fearn S, Trembath DF. 2009. Body size, food habits, reproduction and growth in a population of black whip snakes (Demansia vestigiata) (Serpentes: Elapidae) in tropical Australia. Australian Journal of Zoology 57: 49–54. Fitzgerald M, Shine R, Lemckert F. 2004. Life history attributes of the threatened Australian snake (Stephen’s banded snake Hoplocephalus stephensii, Elapidae). Biological Conservation 119: 121–128. Ford NB, Cobb VA, Lamar WW. 1990. Reproduction data on snakes from northeastern Texas. The Texas Journal of Science 42: 355–367. Ford NB, Karges JP. 1987. Reproduction in the checkered garter snake, Thamnophis marcianus, from Southern Texas and Northeastern Mexico: seasonality and evidence for multiple clutches. The Southwestern Naturalist 32: 93–101. Ford NB, Killebrew DW. 1983. Reproductive tactics and female body size in Butler’s Garter snake, Thamnophis butleri. Journal of Herpetology 17: 271–275. Forsman A, Lindell LE. 1996. Resource dependent growth and body condition dynamics in juvenile snakes: an experiment. Oecologia 108: 669–675. França FGR, Mesquita DO, Nogueira CC, Araújo AFB. 2008. Phylogeny and ecology determine morphological structure in a snake assemblage in the central Brazilian Cerrado. Copeia 2008: 23–38. Gorzula S, Nsiah WO, Oduro W. 1997. Survey of the status and management of the royal python (Python regius) in Ghana. Report to the Secretariat of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), Geneva, Switzerland, 38 p + 6 annexes. Gower DJ, Rasmussen JB, Loader SP, Wilkinson M. 2004. The caecilian amphibian Scolecomorphus kirkii Boulenger as prey of the burrowing asp Atractaspis aterrima (Gunther): trophic relationships of fossorial vertebrates. African Journal of Ecology 42: 83–87. Greene BD, Dixon JR, Whiting MJ, Mueller JM. 1999. Reproductive ecology of the Concho water snake, Nerodia harteri paucimaculata. Copeia 1993: 701–709. Hasegawa M, Moriguchi H. 1989. Geographic variation in food habits, body size and life history traits of the snakes on the Izu Islands. In: Matsui M, Hikida T, Goris RC, eds. Current herpetology in East Asia. Tokyo, Herpetological Society of Japan, 414–432. Hedges SB. 2008. At the lower size limit in snakes: two new species of threadsnakes (Squamata: Leptotyphlopidae: Leptotyphlops) from the Lesser Antilles. Zootaxa 1841: 1–30. Henderson RW, Winstel RA. 1995. Aspects of habitat selection by an arboreal Boa (Corallus enydris) in an area of mixed agriculture on Grenada. Journal of Herpetology 29: 272–275. How AR, Shine R. 1999. Ecological traits and conservation biology of five fossorial ‘sand-swimming’ snake species (Simoselaps: Elapidae) in south-western Australia. Journal of Zoology 249: 269–282. Hoyer RF, Stewart GR. 2000. Biology of the rubber boa (Charina bottae) with emphasis on C. b. umbratica. Part I: Capture, size, sexual dimorphism, and reproduction. Journal of Herpetology 34: 348–354. Inger RF, Zhao E, Bradley SH, Guanfu W. 1990. Report on a collection of amphibians and reptiles from Sichuan, China. Fieldiana: Zoology New Series 58: 1–24. Jacobsen NHG. 1982. The ecology of the reptiles and amphibians in the Burkea africana–Eragrostis pallens savanna of the Nylsvley Nature Reserve. A thesis submitted in partial fulfilment of the requirements for the degree of MSc (Zoology) in the Faculty of Life Sciences. Pretoria: University of Pretoria. Ji X, Wang ZW. 2005. Geographic variation in reproductive traits and trade-offs between size and number of eggs of the Chinese cobra (Naja atra). Biological Journal of the Linnean Society 85: 27–40. Johnson CR. 1975. Thermoregulation in the Papuan-New Guinean boid and colubrid snakes, Candoia carinata, Candoia aspera and Boiga irregularis. Zoological Journal of the Linnean Society 56: 283–290. Kamosawa M, Ota H. 1996. Reproductive biology of the Brahminy Blind Snake (Ramphotyphlops braminus) from the Ryukyu Archipelago, Japan. Journal of Herpetology 30: 9–14. Karns DR, Murphy JC, Voris HK. 2010. Semi-aquatic snake communities of the central plain region of Thailand. Tropical Natural History 10: 1–25. Karns DR, Murphy JC, Voris HK, Suddeth JS. 2005. Comparison of semi-aquatic snake communities associated with the Khorat basin, Thailand. The Natural History Journal of Chulalongkorn University 5: 73–90. SNAKE LENGTH–MASS ALLOMETRY Karns DR, Voris HK, Goodwin TG. 2002. Ecology of Oriental-Australian rear-fanged water snakes (Colubridae: Homalopsinae) in the Pasir Ris Park Mangrove Forest, Singapore. Raffles Bulletin of Zoology 50: 487–498. King RB, Bittner TD, Queral-Regil A, Cline JH. 1999. Sexual dimorphism in neonate and adult snakes. Journal of Zoology 247: 19–28. Labanowski RK, Lowin AJ. 2011. A reptile survey in a dry deciduous forest fragment in northern Madagascar showing new records for the little-known snake Pararhadinaea melanogaster and a range extension for the skink Amphiglossus tanysoma. Herpetology Notes 4: 113–121. Lancaster DL, Ford NB. 2003. Reproduction in western ribbon snakes, Thamnophis proximus (Serpentes: Colubridae), from an east Texas bottomland. Texas Journal of Science 55: 25–32. Lind AJ, Welsh HH. 1990. Predation by Thamnophis couchii on Dicamptodon ensatus. Journal of Herpetology 24: 104– 106. Li-xin S, Shine R, Debi Z, Zhengren T. 2002. Low costs, high output: reproduction in an insular pit-viper (Gloydius shedaoensis, Viperidae) from north-eastern China. Journal of Zoology London 256: 511–521. Lobo A, Pandav P, Vasudevan K. 2004. Weight–length relationships in two species of marine snakes along the coast of Goa, western India. Hamadryad 29: 89–93. Luiselli L, Capula M, Shine R. 1996. Reproductive output, costs of reproduction, and ecology of the smooth snake, Coronella austriaca, in the eastern Italian Alps. Oecologia 106: 100–110. Manjarrez J, Venegas-Barrera CS, Garci’a-Guadarrama T. 2007. Ecology of the Mexican alpine blotched garter snake (Thamnophis scalaris). The Southwestern Naturalist 52: 258–262. Maritz B, Alexander GJ. 2011. Morphology, sexual dimorphism, and growth in the smallest viperid, Bitis schneideri (Reptilia: Squamata: Viperidae). Journal of Herpetology 45: 457–462. Meiri M. 2008. Evolution and ecology of lizard body sizes. Global Ecology and Biogeography 17: 724–734. Moon BR, Candy T. 1997. Coelomic and muscular crosssectional areas in three families of snakes. Journal of Herpetology 31: 37–44. Naulleau G, Girons SH. 1981. Poids des nouveau-nés et reproduction de Vipera aspis (Reptilia: Viperidae), dans des conditions naturelles et artificielles. Amphibia-Reptilia 2: 51–62. Parker WS, Brown WS. 1974. Notes on the ecology of regal ringneck snakes (Diadophis punctatus regalis) in Northern Utah. Journal of Herpetology 8: 262–263. Peabody RB, Johnson JA, Brodie ED Jr. 1975. Intraspecific escape from ingestion of the Rubber Boa, Charina bottae. Journal of Herpetology 9: 237. Pearson D, Shine R, Williams A. 2002. Geographic variation in sexual size dimorphism (Morelia spilota, Pythonidae). Oecologia 131: 418–426. Prival DB, Goode MJ, Swann DE, Schwalbe CR, Schroff MJ. 2002. Natural history of a northern population of 17 twin-spotted rattlesnakes, Crotalus pricei. Journal of Herpetology 36: 598–607. Queral-Regil A, King RB. 1998. Evidence for phenotypic plasticity in snake body size and relative head dimensions in response to amount and size of prey. Copeia 1998: 423–429. Quinn HR, Neitman K. 1978. Reproduction in the Snake Boiga cynodon (Reptilia, Serpentes, Colubridae). Journal of Herpetology 12: 255–256. Rivas JA. 2000. The life history of the green anaconda (Eunectes murinus), with emphasis on its reproductive biology. Doctoral dissertation. Knoxville, TN: University of Tennessee. Rodriguez-Robles JA, Bell CJ, Greene HW. 1999. Gape size and evolution of diet in snakes: feeding ecology of erycine boas. Journal of Zoology 248: 49–58. Schuett GW, Gillingham JC. 1989. Male–male agonistic behavior of the copperhead, Agkistrodon contortrix. Amphibia-Reptilia 10: 243–266. Schumacher J, Lillywhite HB, Norman WM, Jacobson ER. 1997. Effects of ketamine HCl on cardiopulmonary function in snakes. Copeia 1997: 395–400. Schwaner TD, Sarre SD. 1988. Body size of tiger snakes in southern Australia, with particular reference to Notechis ater serventyi (Elapidae) on Chappell Island. Journal of Herpetology 22: 24–33. Secor SM, Nagy TR. 2003. Non-invasive measure of body composition of snakes using dual-energy X-ray absorptiometry. Comparative Biochemistry and Physiology A 136: 379– 389. Seigel RA. 1992. Ecology of a specialized predator – Regina grahami in Missouri. Journal of Herpetology 26: 32–37. Semlitsch RD, Brown KL, Caldwell JP. 1981. Habitat utilization, seasonal activity, and population size structure of the southeastern crowned snake Tantilla coronata. Herpetologica 37: 40–46. Setser K, Mocino-Deloya E, Pleguezuelos JM, Lazcano D, Kardon A. 2010. Reproductive ecology of female Mexican lance-headed rattlesnakes. Journal of Zoology 281: 175–182. Seymor RS. 1987. Scaling of cardiovascular physiology in snakes. American Zoologist 27: 97–109. Shine R. 1991. Strangers in a strange land: ecology of the Australian colubrid Snakes. Copeia 1991: 120–131. Shine R, Branch WR, Harlow PS, Webb JK, Shine T. 2006b. Biology of burrowing Asps (Atractaspididae) from Southern Africa. Copeia 2006: 103–115. Shine R, Branch WR, Webb JK, Harlow PS, Shine T. 2006a. Sexual dimorphism, reproductive biology, and dietary habits of psammophiine snakes (Colubridae) from southern Africa. Copeia 2006: 650–664. Shine R, Fitzgerald M. 1989. Conservation and reproduction of an endangered species: the broad-headed snake, Hoplocephalus bungaroides (Elapidae). Australian Zoologist 25: 65–67. Shine R, Haagner GV, Branch WR, Harlow PS, Webb JK. 1996a. Natural history of the African shieldnose snake Aspidelaps scutatus (Serpentes, Elapidae). Journal of Herpetology 30: 361–366. 18 A. FELDMAN and S. MEIRI Shine R, Harlow PS, Branch WR, Webb JK. 1996b. Life on the lowest branch: sexual dimorphism, diet, and reproductive biology of an African twig snake, Thelotornis capensis (Serpentes, Colubridae). Copeia 1996: 290–299. Shine R, Harlow PS, Keogh JS, Boeadi. 1998. The allometry of life-history traits: insights from a study of giant snakes (Python reticulatus). Journal of Zoology 244: 405– 414. Stevenson DJ, Dyer KJ, Willis-Stevenson BA. 2003. Survey and monitoring of the eastern indigo snake in Georgia. Southern Naturalist 2: 393–408. Tanaka K. 2011. Phenotypic plasticity of body size in an insular population of snake. Herpetologica 67: 46–57. Tanaka K, Ota H. 2002. Natural history of two colubrid snakes, Elaphe quadrivirgata and Rhabdophis tigrinus, on Yakushima Island, southwestern Japan. Amphibia-Reptilia 23: 323–331. Todd BD, Willson JD, Winne CT, Semlitsch RD. 2008. Ecology of the South eastern Crowned Snake, Tantilla coronata. Copeia 2008: 388–394. Trembath DF. 2008. A record of ophiophagy by the spotted python Antaresia maculosa (serpentes: Pythonidae) from Murray Falls National Park, north Queensland, Australia. Herpetofauna 38: 81–83. Van Mierop LHS, Barnard SM. 1976. Observations on the reproduction of Python molurus bivittatus (Reptilia, Serpentes, Boidae). Journal of Herpetology 10: 333–340. Vitt LJ. 1983. Ecology of an anuran-eating guild of terrestrial tropical snakes. Herpetologica 39: 52–66. Vitt LJ, Valdinger LD. 1983. Ecology of a snake community in northern Brazil. Amphibia-Reptilia 4: 273–296. Weatherhead PJ, Barry FE, Brown GR, Forbes MRL. 1995. Sex ratios, mating behavior and sexual size dimorphism of the northern water snake, Nerodia sipedon. Behavioral Ecology and Sociobiology 36: 301–311. Wiley JW. 2003. Habitat association, size, stomach contents, and reproductive condition of Puerto Rican Boas (Epicrates inornatus). Caribbean Journal of Science 39: 189–194. Winne CT, Dorcas ME, Poppy SM. 2005. Population structure, body size, and seasonal activity of black Swamp Snakes (Seminatrix pygaea). Southern Naturalist 4: 1–14. Zuffi MAL, Fornasiero S, Picchiotti R, Rapoli P, Lomele M. 2010. Adaptive significance of food income in European snakes: body size is related to prey energetics. Biological Journal of the Linnean Society 100: 307–317. APPENDIX S2 SPECIES LIST AND SOURCE OF PHYLOGENY, ARRANGED BY FAMILY List of species Boidae Species Source Boa constrictor Calabaria reinhardtii Candoia aspera Charina bottae Corallus caninus Corallus cookii Corallus hortulanus Epicrates cenchria Epicrates inornatus Epicrates maurus Eryx jaculus Eryx johnii Lynch & Wagner (2010) Lynch & Wagner (2010) Lynch & Wagner (2010) Lynch & Wagner (2010) Lynch & Wagner (2010) Lynch & Wagner (2010) Lynch & Wagner (2010) Lynch & Wagner (2010) Lynch & Wagner (2010) Rivera et al. (2011) Lynch & Wagner (2010) Lynch & Wagner (2010) SNAKE LENGTH–MASS ALLOMETRY 19 APPENDIX S2 Continued Colubridae Species Source Species Source Amphiesma sauteri Arizona elegans Atractus badius Atractus pantostictus Bogertophis subocularis Boiga ceylonensis Pyron et al. (2011) Pyron et al. (2011) Vidal et al. (2010a) Franca et al. (2008) Pyron et al. (2011) Kelly et al. (2003) (based on B. dendrophila) Kelly et al. (2003) (based on B. dendrophila) Pyron et al. (2011) Kelly et al. (2003) (based on B. dendrophila) Pyron et al. (2011) Hollis (2006) Hollis (2006) Hollis (2006) Hollis (2006) Hollis (2006) Hollis (2006) Vidal et al. (2010a) Franca et al. (2008) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Vidal et al. (2010a) Vidal et al. (2010a) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Utiger et al. (2002) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011)/ Vidal et al. (2010a) Franca et al. (2008) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Franca et al. (2008) Franca et al. (2008) Vidal et al. (2010a) Liophis typhlus Lygophis meridionalis Lytorhynchus diadema Macroprotodon cucullatus Mussurana quimi Natrix maura Natrix natrix Natrix tessellata Nerodia fasciata Nerodia rhombifer Nerodia sipedon Nerodia taxispilota Opheodrys aestivus Oxybelis aeneus Oxyrhopus formosus Oxyrhopus guibei Oxyrhopus petolarius Oxyrhopus rhombifer Pantherophis guttatus Philodryas aestivus Philodryas nattereri Philodryas olfersii Philodryas patagoniensis Philodryas viridissima Philothamnus semivariegatus Phimophis guerini Phyllorhynchus decurtatus Pituophis melanoleucus Platyceps collaris Platyceps florulentus Platyceps rhodorachis Platyceps rogersi Pseudablabes agassizii Pseudoboa coronata Pseudoboa nigra Ptyas korros Regina grahami Rhabdophis nuchalis Rhabdophis tigrinus Rhinechis scalaris Seminatrix pygaea Sibon nebulatus Spalerosophis diadema Spilotes pullatus Storeria dekayi Storeria occipitomaculata Tantilla melanocephala Vidal et al. (2010a) Franca et al. (2008) Pyron et al. (2011) Pyron et al. (2011) Franca et al. (2008) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Vidal et al. (2010a) Pyron et al. (2011) Pyron et al. (2011) Vidal et al. (2010a) Pyron et al. (2011) Vidal et al. (2010a) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Jesus et al. (2009) Boiga cynodon Boiga dendrophila Boiga irregularis Boiruna maculata Chironius exoletus Chironius flavolineatus Chironius fuscus Chironius multiventris Chironius quadricarinatus Chironius scurrulus Clelia clelia Clelia plumbea Coelognathus radiatus Coluber constrictor Coluber flagellum Coronella austriaca Coronella girondica Dasypeltis scabra Dendrelaphis caudolineatus Dendrophidion dendrophis Diadophis punctatus Dipsas catesbyi Dipsas indica Dipsas variegata Dispholidus typus Dolichophis jugularis Drepanoides anomalus Drymarchon corais Drymobius rhombifer Drymoluber dichrous Eirenis coronelloides Eirenis decemlineatus Eirenis modestus Eirenis rothii Elaphe carinata Elaphe quatuorlineata Elaphe sauromates Erythrolamprus aesculapii Erythrolamprus sp. Helicops angulatus Helicops leopardinus Helicops modestus Hemorrhois hippocrepis Hemorrhois nummifer Heterodon platirhinos Hierophis viridiflavus Imantodes cenchoa Imantodes lentiferus Lampropeltis getula Lampropeltis nigra Leptodeira annulata Leptophis ahaetulla Liophis almadensis Liophis poecilogyrus Liophis reginae Telescopus fallax Thamnodynastes hypoconia Thamnodynastes pallidus Thamnophis butleri Thamnophis couchii Thamnophis marcianus Thamnophis proximus Thamnophis radix Thamnophis scalaris Thelotornis capensis Trimorphodon biscutatus Xenodon merremi Xenodon nattereri Xenopholis scalaris Xenoxybelis argenteus Zamenis longissimus Vidal et al. (2010a) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Vidal et al. (2010a) Vidal et al. (2010a) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Vidal et al. (2000) (genus and Pyron et al., 2011) Pyron et al. (2011) Vidal et al. (2010a) Vidal et al. (2010a) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) de Queiroz et al. (2002) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Franca et al. (2008) Pyron et al. (2011) Zaher et al. (2009) Pyron et al. (2011) 20 A. FELDMAN and S. MEIRI APPENDIX S2 Continued Elapidae Species Source Acanthophis antarcticus Acanthophis pyrrhus Aspidelaps scutatus Bungarus flaviceps Cacophis squamulosus Chitulia inornata Chitulia ornata Cryptophis nigrescens Demansia vestigiata Dendroaspis angusticeps Dendroaspis polylepis Furina diadema Furina tristis Hoplocephalus bungaroides Hoplocephalus stephensii Micrurus frontalis Micrurus ibiboboca Micrurus lemniscatus Naja annulifera Naja mossambica Naja naja Naja nigricollis Notechis scutatus Oxyuranus microlepidotus Oxyuranus scutellatus Pelamis platura Pseudechis australis Pseudonaja affinis Pyron et al. (2011) Reed & Shine (2002) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Lillywhite et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Reed & Shine (2002) Reed & Shine (2002) Reed & Shine (2002) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Reed & Shine (2002) (based on genus) Reed & Shine (2002) (based on genus) Reed & Shine (2002) (based on genus) Pyron et al. (2011) Sanders et al. (2008) Pyron et al. (2011) Sanders et al. (2008) Reed & Shine (2002) Pyron et al. (2011) Reed & Shine (2002) (based on genus) Pyron et al. (2011) Pyron et al. (2011) Pseudonaja nuchalis Pseudonaja textilis Simoselaps bertholdi Simoselaps bimaculatus Simoselaps calonotus Simoselaps fasciolatus Simoselaps littoralis Simoselaps semifasciatus Suta flagellum Tropidechis carinatus Walterinnesia aegyptia Homalopsidae Species Source Cantoria violacea Cerberus australis Cerberus rynchops Enhydris bocourti Enhydris enhydris Enhydris jagorii Enhydris plumbea Enhydris subtaeniata Fordonia leucobalia Gerarda prevostiana Homalopsis buccata Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Voris et al. (2002) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Lamprophiidae Species Source Amblyodipsas polylepis Vidal et al. (2008) (based on genus) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Kelly et al. (2008) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Aparallactus capensis Atractaspis bibronii Atractaspis microlepidota Boaedon fuliginosus Gonionotophis capensis Gonionotophis nyassae Homoroselaps lacteus Lycodonomorphus rufulus Macrelaps microlepidotus Malpolon monspessulanus Psammophis brevirostris Psammophis jallae Psammophis leopardinus Psammophis mossambicus Psammophis notostictus Psammophis schokari Psammophis subtaeniatus Psammophis trigrammus Psammophylax tritaeniatus Pseudaspis cana Pythonidae Species Source Antaresia maculosa Antaresia perthensis Aspidites melanocephalus Aspidites ramsayi Broghammerus reticulatus Liasis fuscus Liasis olivaceus Morelia spilota Morelia viridis Python regius Python sebae Rawlings Rawlings Rawlings Rawlings Rawlings Rawlings Rawlings Rawlings Rawlings Rawlings Rawlings et al. et al. et al. et al. et al. et al. et al. et al. et al. et al. et al. (2008) (2008) (2008) (2008) (2008) (2008) (2008) (2008) (2008) (2008) (2008) SNAKE LENGTH–MASS ALLOMETRY 21 APPENDIX S2 Continued Viperidae Species Source Species Source Agkistrodon bilineatus Agkistrodon contortrix Agkistrodon piscivorus Atheris squamigera Bitis arietans Bitis caudalis Bitis cornuta Bitis gabonica Bitis peringueyi Bitis schneideri Bitis worthingtoni Bothriechis schlegelii Bothriopsis bilineata Bothropoides neuwiedi Bothrops moojeni Causus defilippii Causus resimus Causus rhombeatus Cerastes cerastes Cerastes gasperettii Cerastes vipera Crotalus atrox Crotalus cerastes Crotalus durissus Crotalus horridus Crotalus molossus Crotalus oreganus Crotalus polystictus Crotalus pricei Crotalus ravus Crotalus ruber Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Hampton (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Lenk et al. (2001) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Hampton (2011) Hampton (2011) Pyron et al. (2011) Castoe & Parkinson (2006) Crotalus viridis Daboia palaestinae Daboia russelii Echis carinatus Echis coloratus Echis pyramidum Eristicophis macmahoni Macrovipera deserti Macrovipera lebetina Macrovipera mauritanica Montivipera raddei Montivipera xanthina Ovophis zayuensis Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Malhotra & Thorpe (2004) (based on O. monticola) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Malhotra & Thorpe (2004) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Pyron et al. (2011) Protobothrops jerdonii Pseudocerastes fieldi Pseudocerastes persicus Rhinocerophis alternatus Rhinocerophis itapetiningae Sistrurus catenatus Sistrurus miliarius Trimeresurus kanburiensis Trimeresurus stejnegeri Vipera ammodytes Vipera aspis Vipera berus Vipera latastei Vipera ursinii Scolecophidia Species Source Austrotyphlops australis Austrotyphlops bituberculatus Epictia albipuncta Epictia tenella Leptotyphlops distanti Liotyphlops ternetzii Myriopholis macrorhyncha Ramphotyphlops bicolor Ramphotyphlops braminus Ramphotyphlops polygrammicus Siagonodon septemstriatus Tetracheilostoma breuili Tetracheilostoma carlae Trilepida fuliginosa Trilepida macrolepis Vidal et al. (2010b) Vidal et al. (2010b) Adalsteinsson et al. Adalsteinsson et al. Adalsteinsson et al. Vidal et al. (2010b) Adalsteinsson et al. Vidal et al. (2010b) Vidal et al. (2010b) Vidal et al. (2010b) Adalsteinsson et al. Adalsteinsson et al. Adalsteinsson et al. Adalsteinsson et al. Adalsteinsson et al. (2009), Vidal et al. (2010b) (genus) (2009) (2009) (2009) (2009) (2009) (2009) (2009) (based on genus) (2009) 22 A. FELDMAN and S. MEIRI REFERENCES FOR THE PHYLOGENETIC RELATIONSHIPS Adalsteinsson SA, Branch WR, Trape S, Vitt LJ, Hedges SB. 2009. Molecular phylogeny, classification, and biogeography of snakes of the Family Leptotyphlopidae (Reptilia, Squamata). Zootaxa 2244: 1–50. Castoe TA, Parkinson CL. 2006. Bayesian mixed models and the phylogeny of pitvipers (Viperidae: Serpentes). Molecular Phylogenetics and Evolution 39: 91–110. Franca FGR, Mesquita DO, Nogueira CC, Araujo AFB. 2008. Phylogeny and ecology determine morphological structure in a snake assemblage in the central Brazilian Cerrado. Copeia 2008: 23–28. Hampton PM. 2011. Ventral and sub-caudal scale counts are associated with macrohabitat use and tail specialization in viperid snakes. Evolutionary Ecology 25: 531–546. Hollis JL. 2006. Phylogenetics of the genus Chironius Fitzinger, 1826 (Serpentes, Colubridae) based on morphology. Herpetologica 62: 435–453. Jesus J, Nagy ZT, Branch WR, Wink M, Brehm A, Harris J. 2009. Phylogenetic relationships of African green snakes (genera Philothamnus and Hapsidophrys) from São Tomé, Príncipe and Annobon islands based on mtDNA sequences, and comments on their colonization and taxonomy. Herpetological Journal 19: 41–48. Kelly CMR, Barker NP, Villet MH. 2003. Phylogenetics of advanced snakes (Caenophidia) based on four mitochondrial genes. Systematic Biology 52: 439–459. Kelly CMR, Barker NP, Villet MH, Broadley DG, Branch WR. 2008. The snake family Psammophiidae (Reptilia: Serpentes): phylogenetics and species delimitation in the African sand snakes (Psammophis Boie, 1825) and allied genera. Molecular Phylogenetics and Evolution 47: 1045– 1060. Lenk P, Kalyabina S, Wink M, Joger U. 2001. Evolutionary relationships among the true vipers (Reptilia: Viperidae) inferred from mitochondrial DNA sequences. Molecular Phylogenetics and Evolution 19: 94–104. Lillywhite HB, Albert JS, Sheehy CM III, Seymour RS. 2011. Gravity and the evolution of cardiopulmonary morphology in snakes. Comparative Biochemistry and Physiology – Part A: Molecular and Integrative Physiology 161: 230–242. Lynch VC, Wagner GP. 2010. Did egg-laying boas break Dollo’s law? Phylogenetic evidence for reversal to oviparity in sand boas (Eryx: Boidae). Evolution 64: 207–216. Malhotra A, Thorpe RS. 2004. A phylogeny of four mitochondrial gene regions suggests a revised taxonomy for Asian pitvipers (Trimeresurus and Ovophis). Molecular Phylogenetics and Evolution 32: 83–100. Pyron RA, Burbrinkb FT, Colli GR, Montes de Oca AM, Vitt LJ, Kuczynskia CA, Wiens JJ. 2011. The phylogeny of advanced snakes (Colubroidea), with discovery of a new subfamily and comparison of support methods for likelihood trees. Molecular Phylogenetics and Evolution 58: 329–342. de Queiroz A, Lawson R, Lemos-Espinal JA. 2002. Phylogenetic relationships of North American garter snakes (Thamnophis) based on four mitochondrial genes: how much DNA sequence is enough? Molecular Phylogenetics and Evolution 22: 315–329. Rawlings LH, Rabosky DL, Donnellan SC, Hutchinson MN. 2008. Python phylogenetics: inference from morphology and mitochondrial DNA. Biological Journal of the Linnean Society 93: 603–619. Reed RN, Shine R. 2002. Lying in wait for extinction: ecological correlates of conservation status among Australian elapid snakes. Conservation Biology 16: 451– 461. Rivera PC, Di Cola V, Martınez JJ, Gardenal CN, Chiaraviglio M. 2011. Species delimitation in the continental forms of the genus Epicrates (Serpentes, Boidae) integrating phylogenetics and environmental niche models. PLoS ONE 6: e22199. 10.1371/journal.pone.0022199. Sanders KL, Lee MSY, Leijs R, Foster R, Keogh JS. 2008. Molecular phylogeny and divergence dates for Australasian elapids and sea snakes (Hydrophiinae): evidence from seven genes for rapid evolutionary radiations. Journal of Evolutionary Biology 21: 682–695. Utiger U, Helfenberger N, Schätti B, Schmidt C, Ruf M, Ziswiler V. 2002. Molecular systematics and phylogeny of old and new world ratsnakes, Elaphe Auct., and related genera (Reptilia, Squamata, Colubridae). Russian Journal of Herpetology 9: 105–124. Vidal N, Kindl SG, Wong A, Hedges SB. 2000. Phylogenetic relationships of Xenodontine snakes inferred from 12S and 16S ribosomal RNA sequences. Molecular Phylogenetics and Evolution 14: 389–402. Vidal N, Branch WR, Pauwels OSG, Hedges SB, Broadley DG, Wink M, Cruaud C, Joger U, Nagy ZT. 2008. Dissecting the major African snake radiation: a molecular phylogeny of the Lamprophiidae Fitzinger (Serpentes, Caenophidia). Zootaxa 1945: 51–66. Vidal N, Dewynterb M, Gower DJ. 2010a. Dissecting the major American snake radiation: a molecular phylogeny of the Dipsadidae Bonaparte (Serpentes, Caenophidia). Comptes Rendus Biologies 333: 48–55. Vidal N, Marin J, Morini M, Donnellan S, Branch WR, Thomas R, Vences M, Wynn A, Cruaud C, Hedges SB. 2010b. Blindsnake evolutionary tree reveals long history on Gondwana. Biology Letters 6: 558–561. Voris HK, Alfaro ME, Karns DR, Starnes GL, Thompson E, Murphy JC. 2002. Phylogenetic relationships of the Oriental-Australian rear-fanged water snakes (Colubridae: Homalopsinae) based on mitochondrial DNA sequences. Copeia 2002: 906–915. Zaher H, Grazziotin FG, Cadle JE, Murphy RW, de Moura-Leite JC, Bonatto SL. 2009. Molecular phylogeny of advanced snakes (Serpentes, Caenophidia) with an emphasis on South American Xenodontines: a revised classification and descriptions of new taxa. Papéis Avulsos de Zoologia (São Paulo) 49: 115–153. SNAKE LENGTH–MASS ALLOMETRY 23 APPENDIX S3 PHYLOGENETIC RESULTS, MODEL BY VIDAL ET AL. (2007) General allometries SVL: slope = 2.580 (SE = 0.078), intercept = -5.128 (SE = 0.243), R2 = 0.838. TL: slope = 2.448 (SE = 0.093), intercept = -4.982 (SE = 0.304), R2 = 0.774. The best model for the relationship between mass and SVL included microhabitat use and reproduction mode. This model explained 87.2% of the variation in mass and had a maximum likelihood value of l of 0.45 (significantly different from both zero and unity, P << 0.001). Arboreal species are lighter than the others (P << 0.001 for all cases). Aquatic and burrower species are heavier than terrestrial (P = 0.046 and 0.020, respectively) and terrestrial–arboreal (P = 0.022 and 0.001, respectively) species, but do not differ from each other. Terrestrial and terrestrial–arboreal species do not differ from each other. Viviparous species are heavier than oviparous species (P = 0.014). Parameter estimates The intercept values are for oviparous species in different microhabitats. The value of 0.118 should be added to the intercept values for the calculation of the intercept value of viviparous species in each microhabitat. Intercept Intercept difference -5.708 -5.327 -5.337 -5.500 -5.457 Arboreal Aquatic Burrower Terrestrial–arboreal Terrestrial Viviparous 0.118 APPENDIX S4 EVALUATION OF THE ALLOMETRIES Predictive power of ours and Pough’s allometric equations (number of species in parentheses; first row in each family: P value and t value; second row in each family: percentage of deviation from real mass) Family Pough’s real data New allometries real data Pough’s new allometries All (302) << 0.001, 19.83 0.894, 8.26 << 0.001, 21.93 << 0.001, 17.97 << 0.001, 28.06 0.061, 7.03 0.641, 13.53 0.822, 12.73 0.934, 15.63 0.981, 14.32 0.310, 15.45 0.683, 6.46 0.466 << 0.001, 21.498 0.229 < 0.437, 0.800 Boidae (15) Colubridae (166) Elapidae (26) Lamprophiidae (35) Viperidae (60) View publication stats -13.052 -0.135 -13.792 -4.808 -9.2065 1.906 -0.0827 << 0.001, 32.486 -0.024 << 0.001, 8.773 1.032 << 0.001, 24.175 0.410 << 0.001, -4.35