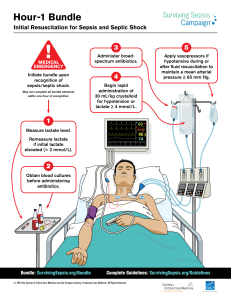
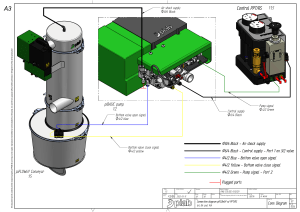
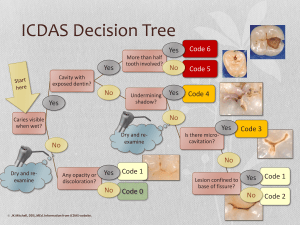
The n e w e ng l a n d j o u r na l of m e dic i n e review article critical care medicine Simon R. Finfer, M.D., and Jean-Louis Vincent, M.D., Ph.D., Editors Circulatory Shock Jean-Louis Vincent, M.D., Ph.D., and Daniel De Backer, M.D., Ph.D. From the Department of Intensive Care, Erasme Hospital, Université Libre de Bruxelles, Brussels. Address reprint requests to Dr. Vincent at the Department of Intensive Care, Erasme University Hospital, Rte. de Lennik 808, B-1070 Brussels, Belgium, or at [email protected]. N Engl J Med 2013;369:1726-34. DOI: 10.1056/NEJMra1208943 Copyright © 2013 Massachusetts Medical Society. S hock is the clinical expression of circulatory failure that ­results in inadequate cellular oxygen utilization. Shock is a common condition in critical care, affecting about one third of patients in the intensive care unit (ICU).1 A diagnosis of shock is based on clinical, hemodynamic, and biochemical signs, which can broadly be summarized into three components. First, systemic arterial hypotension is usually present, but the magnitude of the hypotension may be only moderate, especially in patients with chronic hypertension. Typically, in adults, the systolic arterial pressure is less than 90 mm Hg or the mean arterial pressure is less than 70 mm Hg, with associated tachycardia. Second, there are clinical signs of tissue hypoperfusion, which are apparent through the three “windows” of the body2: cutaneous (skin that is cold and clammy, with vasoconstriction and cyanosis, findings that are most evident in low-flow states), renal (urine output of <0.5 ml per kilogram of body weight per hour), and neurologic (altered mental state, which typically includes obtundation, disorientation, and confusion). Third, hyperlactatemia is typically present, indicating abnormal cellular oxygen metabolism. The normal blood lactate level is approximately 1 mmol per liter, but the level is increased (>1.5 mmol per liter) in acute circulatory failure. Pathoph ysiol o gic a l Mech a nisms An interactive graphic showing initial assessment of shock is available at NEJM.org Shock results from four potential, and not necessarily exclusive, pathophysiological mechanisms3: hypovolemia (from internal or external fluid loss), cardiogenic factors (e.g., acute myocardial infarction, end-stage cardiomyopathy, advanced valvular heart disease, myocarditis, or cardiac arrhythmias), obstruction (e.g., pulmonary embolism, cardiac tamponade, or tension pneumothorax), or distributive factors (e.g., severe sepsis or anaphylaxis from the release of inflammatory mediators) (Fig. 1A and the interactive graphic, available at NEJM.org). The first three mechanisms are characterized by low cardiac output and, hence, inadequate oxygen transport. In distributive shock, the main deficit lies in the periphery, with decreased systemic vascular resistance and altered oxygen extraction. Typically, in such cases cardiac output is high, although it may be low as a result of associated myocardial depression. Patients with acute circulatory failure often have a combination of these mechanisms. For example, a patient with distributive shock from severe pancreatitis, anaphylaxis, or sepsis may also have hypovolemia and cardiogenic shock from myocardial depression. Differ en t i a l Di agnosis Septic shock, a form of distributive shock, is the most common form of shock among patients in the ICU, followed by cardiogenic and hypovolemic shock; ­obstructive shock is relatively rare (Fig. 1B and 1C). In a trial involving more than 1726 n engl j med 369;18 nejm.org october 31, 2013 The New England Journal of Medicine Downloaded from nejm.org on January 8, 2017. For personal use only. No other uses without permission. Copyright © 2013 Massachusetts Medical Society. All rights reserved. Critical Care Medicine 1600 patients with shock who were randomly assigned to receive either dopamine or norepinephrine, septic shock occurred in 62% of the patients, cardiogenic shock in 16%, hypovolemic shock in 16%, other types of distributive shock in 4%, and obstructive shock in 2%.4 The type and cause of shock may be obvious from the medical history, physical examination, or clinical investigations. For example, shock after traumatic injury is likely to be hypovolemic (due to blood loss), but cardiogenic shock or distributive shock may also occur, alone or in combination, caused by such conditions as cardiac tamponade or spinal cord injury. A full clinical examination should include assessment of skin color and temperature, jugular venous distention, and peripheral edema. The diagnosis can be refined with point-of-care echocardiographic evaluation, which includes assessment for pericardial effusion, measurement of left and right ventricular size and function, assessment for respiratory variations in vena cava dimensions, and calculation of the aortic velocity–time integral, a measure of stroke volume. Whenever possible, focused echocardiography should be performed as soon as possible in any patient presenting with shock (Fig. 1A).5,6 Ini t i a l A pproach t o the Pat ien t in Sho ck Ventilatory Support The administration of oxygen should be started immediately to increase oxygen delivery and prevent pulmonary hypertension. Pulse oximetry is often unreliable as a result of peripheral vasoconstriction, and precise determination of oxygen requirements will often require blood gas monitoring. Mechanical ventilation by means of a mask rather than endotracheal intubation has a limited place in the treatment of shock because technical failure can rapidly result in respiratory and cardiac arrest. Hence, endotracheal intubation should be performed to provide invasive mechanical ventilation in nearly all patients with severe dyspnea, hypoxemia, or persistent or worsening acidemia (pH, <7.30). Invasive mechanical ventilation has the additional benefits of reducing the oxygen demand of respiratory muscles and decreasing left ventricular afterload by increasing intrathoracic pressure. An abrupt decrease in arterial pressure after the initiation of invasive mechanical ventilation strongly suggests hypovolemia and a decrease in venous return. The use of sedative agents should be kept to a minimum to avoid further decreases in arterial pressure and cardiac output. Fluid Resuscitation Early, adequate hemodynamic support of patients in shock is crucial to prevent worsening organ dysfunction and failure. Resuscitation should be started even while investigation of the cause is ongoing. Once identified, the cause must be corrected rapidly (e.g., control of bleeding, percutaneous coronary intervention for coronary syndromes, thrombolysis or embolectomy for massive pulmonary embolism, and administration of antibiotics and source control for septic shock). Unless the condition is rapidly reversed, an arterial catheter should be inserted for monitoring of arterial blood pressure and blood sampling, plus a central venous catheter for the infusion of fluids and vasoactive agents and to guide fluid therapy. The initial management of shock is problem-oriented, and the goals are therefore the same, regardless of the cause, although the exact treatments that are used to reach those goals may differ. A useful mnemonic to describe the important components of resuscitation is the VIP rule7: ventilate (oxygen administration), inn engl j med 369;18 fuse (fluid resuscitation), and pump (administration of vasoactive agents). Fluid therapy to improve microvascular blood flow and increase cardiac output is an essential part of the treatment of any form of shock. Even patients with cardiogenic shock may benefit from fluids, since acute edema can result in a decrease in the effective intravascular volume. However, fluid administration should be closely monitored, since too much fluid carries the risk of edema with its unwanted consequences. Pragmatic end points for fluid resuscitation are difficult to define. In general, the objective is for cardiac output to become preload-independent (i.e., on the plateau portion of the Frank– Starling curve), but this is difficult to assess clinically. In patients receiving mechanical ventilation, signs of fluid responsiveness may be identified either directly from beat-by-beat stroke-volume measurements with the use of cardiac-output monitors or indirectly from observed variations in pulse pressure on the arterial-pressure tracing during the ventilator cycle. However, such bedside inferences have some limitations8 — notably, nejm.org october 31, 2013 The New England Journal of Medicine Downloaded from nejm.org on January 8, 2017. For personal use only. No other uses without permission. Copyright © 2013 Massachusetts Medical Society. All rights reserved. 1727 The A n e w e ng l a n d j o u r na l of m e dic i n e B Types of shock Arterial hypotension 62% Distributive (septic) Signs of tissue hypoperfusion Absent Present Brain Altered mental state Chronic hypotension? Syncope (if transient) Circulatory shock Tachycardia Mottled, clammy Normal or high 2% Obstructive 16% 16% Cardiogenic Hypovolemic Estimate cardiac output or SvO2 Elevated blood lactate Skin 4% Distributive (nonseptic) Low Kidney CVP Oliguria Low High Normal cardiac chambers and (usually) preserved contractility Small cardiac chambers and normal or high contractility Large ventricles and poor contractility In tamponade: pericardial effusion, small right and left ventricles, dilated inferior vena cava; in pulmonary embolism or pneumothorax: dilated right ventricle, small left ventricle Distributive shock Hypovolemic shock Cardiogenic shock Obstructive shock Distributive shock Hypovolemic shock Cardiogenic shock Obstructive shock Echocardiography C Vasodilatation Loss of plasma or blood volume Obstruction Ventricular failure Pericardial tamponade Figure 1. Initial Assessment of Shock States. COLOR FIGURE Shown is an algorithm for the initial assessment of a patient in shock (Panel A), relative frequencies of the main types of shock (Panel B), Draft 9presentation 10/10/13 and schematic representations of the four main types of shock (Panel C). The algorithm starts with the most common Author Vincent (i.e., arterial hypotension), but hypotension is sometimes minimal or absent. CVP denotes central venous pressure, and SvO2 mixed 1 Fig # venous oxygen saturation. Title ME Drazen Knoper DE Artist AUTHOR PLEASE NOTE: 1728 n engl j med 369;18 nejm.org october 31, 2013 Figure has been redrawn and type has been reset Please check carefully Issue date 10/31/13 The New England Journal of Medicine Downloaded from nejm.org on January 8, 2017. For personal use only. No other uses without permission. Copyright © 2013 Massachusetts Medical Society. All rights reserved. Critical Care Medicine a vasopressor temporarily while fluid resuscitation is ongoing, with the aim of discontinuing it, if possible, after hypovolemia has been corrected. Adrenergic agonists are the first-line vasopressors because of their rapid onset of action, high potency, and short half-life, which allows easy dose adjustment. Stimulation of each type of adrenergic receptor has potentially beneficial and harmful effects. For example, β-adrenergic stimulation can increase blood flow but also increases the risk of myocardial ischemia as a result of increased heart rate and contractility. Hence, the use of isoproterenol, a pure β-adrenergic agent, is limited to the treatment of patients with severe bradycardia. At the other extreme, α-adrenergic stimulation will increase vascular tone and blood pressure but can also decrease cardiac output and impair tissue blood flow, especially in the hepatosplanchnic region. For this reason, phenyl­ ephrine, an almost pure α-adrenergic agent, is rarely indicated. We consider norepinephrine to be the vasopressor of first choice; it has predominantly α-adren­ergic properties, but its modest β-adrener­ gic effects help to maintain cardiac output. Administration generally results in a clinically significant increase in mean arterial pressure, with little change in heart rate or cardiac output. The usual dose is 0.1 to 2.0 µg per kilogram of body weight per minute. Dopamine has predominantly β-adrenergic effects at lower doses and α-adrenergic effects at higher doses, but its effects are relatively weak. Dopaminergic effects at very low doses (<3 µg per kilogram per minute, given intravenously) may selectively dilate the hepatosplanchnic and renal circulations, but controlled trials have not shown a protective effect on renal function,14 and its routine use for this purpose is no longer recommended. Dopaminergic stimulation may also have undesired endocrine effects on the hypothalamic–pituitary system, resulting in immuno­ suppression, primarily through a reduction in the release of prolactin. In a recent randomized, controlled, doubleblind trial, dopamine had no advantage over nor­ Vasoactive Agents epinephrine as the first-line vasopressor agent; Vasopressors moreover, it induced more arrhythmias and was If hypotension is severe or if it persists despite associated with an increased 28-day rate of fluid administration, the use of vasopressors is death among patients with cardiogenic shock.4 indicated. It is acceptable practice to administer Administration of dopamine, as compared with that the patient must receive ventilation with relatively large tidal volumes, have no spontaneous breathing effort (which usually requires the administration of sedatives or even muscle relaxants), and be free of major arrhythmia and right ventricular dysfunction. A passive leg-raising test is an alternative method9 but requires a rapidresponse device, since the effect is transient. Regardless of the test used, there remains a gray zone in which it is difficult to predict a patient’s response to intravenous fluids. A fluid-challenge technique should be used to determine a patient’s actual response to fluids, while limiting the risks of adverse effects. A fluid challenge incorporates four elements that should be defined in advance.10 First, the type of fluid must be selected. Crystalloid solutions are the first choice, because they are well tolerated and cheap. The use of albumin to correct severe hypoalbuminemia may be reasonable in some patients.11 (A detailed examination of the choice of resuscitation fluids was provided in a previous article in this series12 and thus is not included in this review.) Second, the rate of fluid administration must be defined. Fluids should be infused rapidly to induce a quick response but not so fast that an artificial stress response develops; typically, an infusion of 300 to 500 ml of fluid is administered during a period of 20 to 30 minutes.13 Third, the objective of the fluid challenge must be defined. In shock, the objective is usually an increase in systemic arterial pressure, although it could also be a decrease in heart rate or an increase in urine output. Finally, the safety limits must be defined. Pulmonary edema is the most serious complication of fluid infusion. Although it is not a perfect guideline, a limit in central venous pressure of a few millimeters of mercury above the baseline value is usually set to prevent fluid overload.13 Stimulation of the patient and any other change in therapy should be avoided during the test. Fluid challenges can be repeated as required but must be stopped rapidly in case of non­ response in order to avoid fluid overload. n engl j med 369;18 nejm.org october 31, 2013 The New England Journal of Medicine Downloaded from nejm.org on January 8, 2017. For personal use only. No other uses without permission. Copyright © 2013 Massachusetts Medical Society. All rights reserved. 1729 The n e w e ng l a n d j o u r na l norepinephrine, may also be associated with higher rates of death among patients with septic shock.15 Hence, we no longer recommend dopamine for the treatment of patients with shock. Epinephrine, which is a stronger agent, has predominantly β-adrenergic effects at low doses, with α-adrenergic effects becoming more clinically significant at higher doses. However, epinephrine administration can be associated with an increased rate of arrhythmia16,17 and a decrease in splanchnic blood flow16 and can increase blood lactate levels, probably by increasing cellular metabolism.16,18 Prospective, randomized studies have not shown any beneficial effects of epinephrine over norepinephrine in septic shock.17,18 We reserve epinephrine as a second-line agent for severe cases.13 The use of other strong vasopressor agents as continuous infusions (e.g., angiotensin or metaraminol) has largely been abandoned. Nonselective inhibition of nitric oxide has not been shown to be beneficial in patients with cardiogenic shock19 and is detrimental in patients with septic shock.20 Vasopressin deficiency can develop in patients with very hyperkinetic forms of distributive shock, and the administration of low-dose vasopressin may result in substantial increases in arterial pressure. In the Vasopressin and Septic Shock Trial (VASST), investigators found that the addition of low-dose vasopressin to norepinephrine in the treatment of patients with septic shock was safe21 and may have been associated with a survival benefit for patients with forms of shock that were not severe and for those who also received glucocorticoids.22 Vasopressin should not be used at doses higher than 0.04 U per minute and should be administered only in patients with a high level of cardiac output. Terlipressin, an analogue of vasopressin, has a duration of action of several hours, as compared with minutes for vasopressin. For this reason, we do not believe it offers an advantage over vasopressin in the ICU. Vasopressin derivatives with more selective V1-receptor activity are currently being studied. Inotropic Agents We consider dobutamine to be the inotropic agent of choice for increasing cardiac output, regardless of whether norepinephrine is also being given. With predominantly β-adrenergic proper1730 n engl j med 369;18 of m e dic i n e ties, dobutamine is less likely to induce tachycardia than isoproterenol. An initial dose of just a few micrograms per kilogram per minute may substantially increase cardiac output. Intravenous doses in excess of 20 µg per kilogram per minute usually provide little additional benefit. Dobutamine has limited effects on arterial pressure, although pressure may increase slightly in patients with myocardial dysfunction as the primary abnormality or may decrease slightly in patients with underlying hypovolemia. Instead of routine administration of a fixed dose of dobutamine to increase oxygen delivery to supranormal, predetermined levels, the dose should be adjusted on an individual basis to achieve adequate tissue perfusion. Dobutamine may improve capillary perfusion in patients with septic shock, independent of its systemic effects.23 Phosphodiesterase type III inhibitors, such as milrinone and enoximone, combine inotropic and vasodilating properties. By decreasing the metabolism of cyclic AMP, these agents may reinforce the effects of dobutamine. They may also be useful when β-adrenergic receptors are downregulated or in patients recently treated with beta-blockers. However, phosphodiesterase type III inhibitors may have unacceptable adverse effects in patients with hypotension, and the long half-lives of these agents (4 to 6 hours) prevent minute-to-minute adjustment. Hence, intermittent, short-term infusions of small doses of phosphodiesterase III inhibitors may be preferable to a continuous infusion in shock states. Levosimendan, a more expensive agent, acts primarily by binding to cardiac troponin C and increasing the calcium sensitivity of myocytes, but it also acts as a vasodilator by opening ATPsensitive potassium channels in vascular smooth muscle. However, this agent has a half-life of several days, which limits the practicality of its use in acute shock states. Vasodilators By reducing ventricular afterload, vasodilating agents may increase cardiac output without increasing myocardial demand for oxygen. The major limitation of these drugs is the risk of decreasing arterial pressure to a level that compromises tissue perfusion. Nevertheless, in some patients, prudent use of nitrates and possibly other vasodilators may improve microvascular perfusion and cellular function.24 nejm.org october 31, 2013 The New England Journal of Medicine Downloaded from nejm.org on January 8, 2017. For personal use only. No other uses without permission. Copyright © 2013 Massachusetts Medical Society. All rights reserved. Critical Care Medicine Mech a nic a l Supp or t A Mechanical support with intraaortic balloon counterpulsation (IABC) can reduce left ventricular afterload and increase coronary blood flow. However, a recent randomized, controlled trial showed no beneficial effect of IABC in patients with cardiogenic shock,25 and its routine use in cardiogenic shock is not currently recommended. Venoarterial extracorporeal membrane oxygenation (ECMO) may be used as a temporary lifesaving measure in patients with reversible cardiogenic shock or as a bridge to heart transplantation.26 G oa l s of Hemody na mic Supp or t B Arterial Pressure The primary goal of resuscitation should be not only to restore blood pressure but also to provide adequate cellular metabolism, for which the correction of arterial hypotension is a prerequisite. Restoring a mean systemic arterial pressure of 65 to 70 mm Hg is a good initial goal, but the level should be adjusted to restore tissue perfusion, assessed on the basis of mental status, skin appearance, and urine output, as described above. In patients with oliguria, in particular, the effects of a further increase in arterial pressure on urine output should be assessed regularly, unless acute renal failure is already established. Conversely, a mean arterial pressure lower than 65 to 70 mm Hg may be acceptable in a patient with acute bleeding who has no major neurologic problems, with the aim of limiting blood loss and associated coagulopathy, until the bleeding is controlled. Cardiac Output and Oxygen Delivery Since circulatory shock represents an imbalance between oxygen supply and oxygen requirements, maintaining adequate oxygen delivery to the tissues is essential, but all the strategies to achieve this goal have limitations. After correction of hypoxemia and severe anemia, cardiac output is the principal determinant of oxygen delivery, but the optimal cardiac output is difficult to define. Cardiac output can be measured by means of various techniques, each of which has its own benefits and drawbacks.6 Absolute measures of cardiac output are less important than monitoring trends in response to interventions such as a fluid challenge. The targeting of a predefined cardiac output is not advisable, since the cardiac output that n engl j med 369;18 Figure 2. Sidestream Dark-Field Images of Sublingual Microcirculation in a Healthy Volunteer and a Patient with Septic Shock. The microcirculation in the healthy volunteer is characterized by dense capillaries that are consistently perfused (Panel A, arrows), whereas in the patient with septic shock, the density of the capillaries is diminished, and many of the capillaries have stopped or intermittent flow (Panel B, arrows). is needed will vary among patients and in the same patient over time. Measurements of mixed venous oxygen saturation (SvO2) may be helpful in assessing the adequacy of the balance between oxygen demand and supply; SvO2 measurements are also very useful in the interpretation of cardiac output.27 SvO2 is typically decreased in patients with low-flow states or anemia but is normal or high in those with distributive shock. Its surrogate, central venous oxygen saturation (ScvO2), which is measured in the superior vena cava by means of a central venous catheter, reflects the oxygen saturation of the venous blood from the upper half of the body only. Under normal circumstances, ScvO2 is slightly less than SvO2, but in critically ill patients it is often greater. Rivers et al.28 found nejm.org october 31, 2013 The New England Journal of Medicine Downloaded from nejm.org on January 8, 2017. For personal use only. No other uses without permission. Copyright © 2013 Massachusetts Medical Society. All rights reserved. 1731 The n e w e ng l a n d j o u r na l of m e dic i n e Phase Focus Microcirculatory Variables Salvage Optimization Stabilization De-escalation Obtain a minimal acceptable blood pressure Provide adequate oxygen availability Provide organ support Wean from vasoactive agents Perform lifesaving measures Optimize cardiac output, SvO2, lactate Minimize complications Achieve a negative fluid balance Figure 3. Four Phases in the Treatment of Shock. COLOR FIGURE The salvage phase focuses on achieving a blood pressure and cardiac outDraft 6 10/15/13 put compatible with immediate survival and performing lifesaving proceAuthor Vincent dures to treat the underlying cause of shock. The Fig optimization phase focus3 # Title es on promoting cellular oxygen availability and monitoring cardiac output, mixed venous oxygen saturation (SvO2), and lactate levels. The stabilization ME phase focuses on preventing organ dysfunction, even after hemodynamic DE Drazen Artist focuses Knoper on weaning stability has been achieved. The de-escalation phase AUTHOR PLEASE NOTE: the patient from vasoactive agents and providing treatments to help Figure has been redrawn and type has been reset Please check carefully achieve a negative fluid balance. Issue date 10/31/13 that in patients presenting to the emergency department with septic shock, a treatment algorithm targeting an ScvO2 of at least 70% during the first 6 hours was associated with decreased rates of death. The robustness of this finding is currently being evaluated in three multicenter trials. (ClinicalTrials.gov numbers, NCT00975793 and NCT00510835; and Current Controlled Trials number, ISRCTN36307479). The development of handheld devices for orthogonal polarization spectral (OPS) imaging and its successor, sidestream dark-field (SDF) imaging, is providing new means of directly visualizing the microcirculation and evaluating the effects of interventions on microcirculatory flow in easily accessible surfaces, such as the sublingual area.30 Microcirculatory changes, including decreased capillary density, a reduced proportion of perfused capillaries, and increased heterogeneity of blood flow, have been identified in various types of circulatory shock (Fig. 2), and the persistence of these alterations is associated with worse outcomes.31 Near-infrared spectroscopy is a technique that uses near-infrared light to determine tissue oxygen saturation from the fractions of oxyhemoglobin and deoxyhemoglobin. Analysis of the changes in tissue oxygen saturation during a brief episode of forearm ischemia can be used to quantify microvascular dysfunction32; such alterations are associated with worse outcomes.33 Various therapeutic interventions have been shown to have an effect on these microcirculatory variables, but whether therapy that is guided by monitoring or targeting the microcirculation can improve outcomes requires further study and cannot be recommended at this time. Ther a peu t ic Pr ior i t ie s a nd G oa l s Blood Lactate Level An increase in the blood lactate level reflects abnormal cellular function. In low-flow states, the primary mechanism of hyperlactatemia is tissue hypoxia with development of anaerobic metabolism, but in distributive shock, the pathophysiology is more complex and may also involve increased glycolysis and inhibition of pyruvate dehydrogenase. In all cases, alterations in clearance can be due to impaired liver function. The value of serial lactate measurements in the management of shock has been recognized for 30 years.29 Although changes in lactate take place more slowly than changes in systemic arterial pressure or cardiac output, the blood lactate level should decrease over a period of hours with effective therapy. In patients with shock and a blood lactate level of more than 3 mmol per liter, Jansen et al.24 found that targeting a decrease of at least 20% in the blood lactate level over a 2-hour period seemed to be associated with reduced in-hospital mortality. 1732 n engl j med 369;18 There are essentially four phases in the treatment of shock, and therapeutic goals and monitoring need to be adapted to each phase (Fig. 3). In the first (salvage) phase, the goal of therapy is to achieve a minimum blood pressure and cardiac output compatible with immediate survival. Minimal monitoring is needed; in most cases, invasive monitoring can be restricted to arterial and central venous catheters. Lifesaving procedures (e.g., surgery for trauma, pericardial drainage, revascularization for acute myocardial infarction, and antibiotics for sepsis) are needed to treat the underlying cause. In the second (optimization) phase, the goal is to increase cellular oxygen availability, and there is a narrow window of opportunity for interventions targeting hemodynamic status.28 Adequate hemodynamic resuscitation reduces inflammation, mitochondrial dysfunction, and caspase activation.34,35 Measurements of SvO2 and lactate levels may help guide therapy, and monitoring of cardiac output should be considered. In the third nejm.org october 31, 2013 The New England Journal of Medicine Downloaded from nejm.org on January 8, 2017. For personal use only. No other uses without permission. Copyright © 2013 Massachusetts Medical Society. All rights reserved. Critical Care Medicine (stabilization) phase, the goal is to prevent organ dysfunction, even after hemodynamic stability has been achieved. Oxygen supply to the tissues is no longer the key problem, and organ support becomes more relevant. Finally, in the fourth (deescalation) phase, the goal is to wean the patient from vasoactive agents and promote spontaneous polyuria or provoke fluid elimination through the use of diuretics or ultrafiltration to achieve a negative fluid balance. C onclusions Circulatory shock is associated with high morbidity and mortality. Prompt identification is es- sential so that aggressive management can be started. Appropriate treatment is based on a good understanding of the underlying pathophysiological mechanisms. Treatment should include correction of the cause of shock and hemodynamic stabilization, primarily through fluid infusion and administration of vasoactive agents. The patient’s response can be monitored by means of careful clinical evaluation and blood lactate measurements; microvascular evaluation may be feasible in the future. No potential conflict of interest relevant to this article was reported. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. References 1. Sakr Y, Reinhart K, Vincent JL, et al. Does dopamine administration in shock influence outcome? Results of the Sepsis Occurrence in Acutely Ill Patients (SOAP) Study. Crit Care Med 2006;34:589-97. 2. Vincent JL, Ince C, Bakker J. Circulatory shock — an update: a tribute to Professor Max Harry Weil. Crit Care 2012; 16:239. 3. Weil MH, Shubin H. Proposed reclassification of shock states with special reference to distributive defects. Adv Exp Med Biol 1971;23:13-23. 4. De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010;362:779-89. 5. Labovitz AJ, Noble VE, Bierig M, et al. Focused cardiac ultrasound in the emergent setting: a consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr 2010;23: 1225-30. 6. Vincent JL, Rhodes A, Perel A, et al. Clinical review: update on hemodynamic monitoring — a consensus of 16. Crit Care 2011;15:229. 7. Weil MH, Shubin H. The “VIP” approach to the bedside management of shock. JAMA 1969;207:337-40. 8. Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med 2009;37:2642-7. 9. Cavallaro F, Sandroni C, Marano C, et al. Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: systematic review and metaanalysis of clinical studies. Intensive Care Med 2010;36:1475-83. 10. Vincent JL, Weil MH. Fluid challenge revisited. Crit Care Med 2006;34:1333-7. 11. Delaney AP, Dan A, McCaffrey J, Finfer S. The role of albumin as a resuscita- tion fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med 2011;39:386-91. 12. Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med 2013;369:124351. 13. Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580-637. 14. Bellomo R, Chapman M, Finfer S, Hickling K, Myburgh J. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Lancet 2000;356:2139-43. 15. De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a metaanalysis. Crit Care Med 2012;40:725-30. 16. Levy B, Perez P, Perny J, Thivilier C, Gerard A. Comparison of norepinephrinedobutamine to epinephrine for hemo­ dynamics, lactate metabolism, and organ function variables in cardiogenic shock: a prospective, randomized pilot study. Crit Care Med 2011;39:450-5. 17. Annane D, Vignon P, Renault A, et al. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet 2007;370:676-84. [Erratum, Lancet 2007; 370:1034.] 18. Myburgh JA, Higgins A, Jovanovska A, Lipman J, Ramakrishnan N, Santamaria J. A comparison of epinephrine and norepinephrine in critically ill patients. Intensive Care Med 2008;34:2226-34. 19. Alexander JH, Reynolds HR, Stebbins AL, et al. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: the TRIUMPH randomized controlled trial. JAMA 2007; 297:1657-66. 20. López A, Lorente JA, Steingrub J, et al. Multiple-center, randomized, placebocontrolled, double-blind study of the ni- n engl j med 369;18 nejm.org tric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med 2004;32:21-30. 21. Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 2008;358:877-87. 22. Russell JA, Walley KR, Gordon AC, et al. Interaction of vasopressin infusion, corticosteroid treatment, and mortality of septic shock. Crit Care Med 2009;37:811-8. 23. De Backer D, Creteur J, Dubois MJ, et al. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med 2006;34: 403-8. 24. Jansen TC, van Bommel J, Schoon­ derbeek FJ, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med 2010;182:752-61. 25. Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287-96. 26. Combes A, Leprince P, Luyt CE, et al. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med 2008;36: 1404-11. 27. Vincent JL. Understanding cardiac output. Crit Care 2008;12:174. 28. Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368-77. 29. Vincent JL, Dufaye P, Berré J, Leeman M, Degaute JP, Kahn RJ. Serial lactate determinations during circulatory shock. Crit Care Med 1983;11:449-51. 30. De Backer D, Hollenberg S, Boerma C, et al. How to evaluate the microcirculation: report of a round table conference. Crit Care 2007;11:R101. october 31, 2013 The New England Journal of Medicine Downloaded from nejm.org on January 8, 2017. For personal use only. No other uses without permission. Copyright © 2013 Massachusetts Medical Society. All rights reserved. 1733 Critical Care Medicine 31. Sakr Y, Dubois MJ, De Backer D, ­ reteur J, Vincent JL. Persistent micro­ C circulatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 2004;32: 1825-31. 32. Gómez H, Torres A, Polanco P, et al. Use of non-invasive NIRS during a vascular occlusion test to assess dynamic tissue 1734 O(2) saturation response. Intensive Care Med 2008;34:1600-7. 33. Creteur J, Carollo T, Soldati G, Buchele G, De Backer D, Vincent JL. The prognostic value of muscle StO2 in septic patients. Intensive Care Med 2007;33:1549-56. 34. Rivers EP, Kruse JA, Jacobsen G, et al. The influence of early hemodynamic optimization on biomarker patterns of severe n engl j med 369;18 nejm.org sepsis and septic shock. Crit Care Med 2007;35:2016-24. 35. Corrêa TD, Vuda M, Blaser AR, et al. Effect of treatment delay on disease severity and need for resuscitation in porcine fecal peritonitis. Crit Care Med 2012;40: 2841-9. Copyright © 2013 Massachusetts Medical Society. october 31, 2013 The New England Journal of Medicine Downloaded from nejm.org on January 8, 2017. For personal use only. No other uses without permission. Copyright © 2013 Massachusetts Medical Society. All rights reserved.