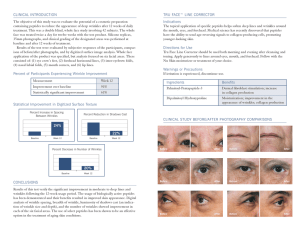
Progress in Lipid Research 51 (2012) 149–177 Contents lists available at SciVerse ScienceDirect Progress in Lipid Research journal homepage: www.elsevier.com/locate/plipres Review Role of lipids in the interaction of antimicrobial peptides with membranes Vitor Teixeira a,b,⇑, Maria J. Feio b, Margarida Bastos a a b CIQ(UP), Departamento de Química e Bioquímica, FCUP, R. do Campo Alegre, Porto 4169-007, Portugal REQUIMTE, Departamento de Química e Bioquímica, FCUP, R. do Campo Alegre, Porto 4169-007, Portugal a r t i c l e i n f o Article history: Available online 8 January 2012 Keywords: Antimicrobial peptide Immunity Structure–activity relationship Peptide design Target activity Phospholipids Mechanism of action Membrane permeabilization Membrane topology Intracellular targets Cell death Resistance Therapeutic agents a b s t r a c t Antimicrobial peptides (AMPs) take part in the immune system by mounting a first line of defense against pathogens. Recurrent structural and functional aspects are observed among peptides from different sources, particularly the net cationicity and amphipathicity. However, the membrane seems to be the key determinant of their action, either as the main target of the peptide action or by forming a barrier that must be crossed by peptides to target core metabolic pathways. More importantly, the specificity exhibited by antimicrobial peptides relies on the different lipid composition between pathogen and host cells, likely contributing to their spectrum of activity. Several mechanisms of action have been reported, which may involve membrane permeabilization through the formation of pores, membrane thinning or micellization in a detergent-like way. AMPs may also target intracellular components, such as DNA, enzymes and even organelles. More recently, these peptides have been shown to produce membrane perturbation by formation of specific lipid–peptide domains, lateral phase segregation of zwitterionic from anionic phospholipids and even the formation of non-lamellar lipid phases. To countermeasure their activity, some pathogens were successful in developing effective mechanisms of resistance to decrease their susceptibility to AMPs. The functional and integral knowledge of such interactions and the clarification of the complex interplay between molecular determinants of peptides, the pathogen versus host cells dichotomy and the specific microenvironment in which all these elements convene will contribute to an understanding of some elusive aspects of their action and to rationally design novel therapeutic agents to overcome the current antibiotic resistance issue. Ó 2012 Elsevier Ltd. All rights reserved. Contents 1. 2. 3. 4. 5. Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Antimicrobial peptide structural diversity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.1. Classic small peptides . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.1.1. Amphipathic a-helical peptides. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.1.2. Amphipathic b-sheet peptides . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.1.3. Other and specific residue-rich peptides. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.2. Larger antimicrobial proteins and polypeptides . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Biological significance of membrane composition as a measure of affinity to antimicrobial peptides. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Molecular determinants of antimicrobial peptides . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4.1. Sequence and Structure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4.2. Charge . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4.3. Amphipathicity and hydrophobic moment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4.4. Hydrophobicity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4.5. Polar angle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Antimicrobial peptide’s mechanisms of action: an overall perspective . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.1. Adsorption and binding to membranes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.2. Threshold concentration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 150 151 151 151 151 152 152 152 154 154 155 155 156 156 156 157 158 ⇑ Corresponding author at: REQUIMTE, Departamento de Química e Bioquímica, FCUP, R. do Campo Alegre, Porto 4169-007, Portugal. Tel.: +351 22 0402511; fax: +351 22 0402659. E-mail address: [email protected] (V. Teixeira). 0163-7827/$ - see front matter Ó 2012 Elsevier Ltd. All rights reserved. doi:10.1016/j.plipres.2011.12.005 150 V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 5.3. 5.4. 6. 7. 8. 9. Conformational transition. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Peptide insertion and membrane permeability . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.4.1. Membranolytic activity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.4.2. Non-membranolytic activity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Intracellular targets of antimicrobial peptides: an alternative mechanism of action? . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Mechanisms of antimicrobial peptide resistance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7.1. Membrane electrostatics and structural modifications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7.2. Membrane electrical potential . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7.3. Sensor-transducer response systems . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7.4. Proteases and peptidases . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7.5. Efflux-dependent resistance mechanisms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Development of antimicrobial peptides for clinical applications: a novel advance in therapeutics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8.1. A promising future for antimicrobial peptides as reliable antibiotics? . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Concluding remarks and future directions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Acknowledgments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1. Introduction The integrity of any eukaryotic organism depends not only on the proper expression of its genes but also on its ability to resist the action from invading microorganisms. It demands a structured and a functional immune system in constant interaction with the dynamic surrounding environment which in turn determines the ability of the host to prevent an infection. In order to establish an infection, a pathogen must first overcome many surface barriers, such as physical and mechanical elements like skin, mucous membranes and the epithelia of the respiratory system or the gastrointestinal and genitourinary tracts [1]. In addition, there are also important chemical mediators of the immune system that constitute an effective arsenal to overcome the harmful action of microorganisms. Some of these chemicals and molecules include gastric juices, salivary glycoproteins, lysozyme, antimicrobial peptides, the complement system, cytokines and acute-phase proteins which possess antiviral, antifungal, antitumoral and immunomodulatory activities [1–3]. Antimicrobial peptides (AMPs) are a universal feature of the defense systems of virtually all forms of life, with representatives found in organisms ranging from bacteria to plants, fish, amphibians, insects, mammals, and even viruses [1,4–15]. They take part in an ancient, nonspecific innate immune system, which is the main defense mechanism for the majority of living organisms during the initial stages of an infection [1,16,17]. These peptides usually display a broad range activity as they act on bacteria, fungi, metazoans and other parasites, viruses and even cancer cells [18,19]. The importance of these mechanisms to host defense may vary between different sites (skin, oral cavity, gastrointestinal tract, respiratory system) within a particular organism and even between different organisms [1]. Antimicrobial peptides may be expressed constitutively or can be inducibly expressed in response to exposure to foreign microorganisms. Indeed, they may be expressed systemically, as observed for cecropins isolated from the hemolymph of bacteria-challenged moths and flies of the Lepidoptera and Diptera orders. They can even be localized in specific cell or tissue types in the body, most susceptible to an infection from a particular set of pathogens. For instance, histatins are the main representatives on major salivary glands in humans with bactericidal and fungicidal activities that contribute to the innate defense of the oral cavity and more recently as oral wound healing factors [20–22]. Plant defensins are more abundant on the epidermal cell layer and leaf primordial of the potato tuber, which is consistent with a role in first-line defense of vulnerable tissues [23]. Such compartmentalization has proved to be important according to the type and specificity of the invading organism, which naturally implies not only 158 159 159 161 167 168 168 169 170 170 171 171 172 172 172 172 cell-specific regulation of expression but also a polarity in their distribution on the organism. Antimicrobial peptides have typically 12–50 amino acids, a molecular mass less than 10 kDa, and possess 2–9 positively charged lysine or arginine residues that confer their overall positive net charge at physiological pH. Only a small number of acidic residues (aspartate and glutamate) are usually found in these peptides, presumably contributing to the increase of amphipathicity when present on the polar face [7,10,14,24]. Moreover, these immunity peptide mediators usually present up to 50% hydrophobic amino acids, contributing to the common amphipatic conformation that they tend to assume at the lipid membrane interface when interacting with the target cell [1,11,24–27]. Although these peptides are mostly recognized by their antibacterial activity, many studies have pointed out an increasingly recognized function as modulators of the innate immune response in higher organisms (Fig. 1) [1,2,28–30]. In fact, a number of properties that modulate the immune response have been attributed to these host defense peptides in many organisms, particularly in mammals. These include epithelial cell proliferation, enhanced wound healing, angiogenesis and the stimulation of chemokine production, regulation of the production of pro-inflammatory cytokines and direct chemotaxis of expression of many types of leukocytes [1] (Fig. 1). It has been known that many cationic peptides, Fig. 1. Biological functions of antimicrobial peptides in immunity. Antimicrobial peptides are mostly recognized by their antibacterial activity (mainly mediated by membrane permeabilization and cell death) but many studies have provided evidence of their immunomodulatory functions in infection, such as chemotaxis, angiogenesis and regulation of the magnitude of the adaptive immune response by modulating the Th cell polarization. V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 including human cathelicidin LL-37 [31] and CP26 [28], are able to neutralize endotoxins (e.g. LPS), both in vitro (inhibiting LPS-induced TNF-a production in macrophages) and in vivo, by protecting animals against endotoxemia. Cationic host defence peptides derived from a range of other sources, including the bovine cathelicidin BMAP-27 [32], indolicidin [33], insect and bee-derived cecropin–melittin hybrid peptides [34], and other synthetic cationic peptides [35], have been shown to contribute to a significant reduction of endotoxin-induced inflammatory responses. This apparent convergence of action, though from different sources, suggests that the anti-endotoxin activity exhibited by these peptides may be conserved across species. It is clear that host defence peptides have the potential to modulate the innate immune response through a wide variety of mechanisms, but recent results suggest that these peptides may also play a role in modulating and bridging the adaptive immune response elements. Some studies have demonstrated that different defense effector molecules working in synergy with cationic defense peptides results in an increased clearance of invading pathogens [36]. In vitro studies with human defensins have demonstrated that they can enhance cellular proliferation and cytokine responses of CD4+ T cells through IFN-c and IL-10 induction, as well as modulation of the expression of co-stimulatory molecules [37]. This provides evidence for peptide involvement in humoral (Th2-dependent) responses, and suggests that host defense peptides may possess adjuvant-like properties [38]. Similarly, human a-defensins and murine b-defensins can stimulate Th1-dependent cellular responses mainly represented by antigen-specific cytotoxic T lymphocytes, and enhance anti-tumoral and anti-viral immunity [39]. Indeed, such antiviral action by antimicrobial peptides cecropin, melittin and indolicidin has already been demonstrated for a few viruses, such as murine leukaemia viruses, feline immunodeficiency viruses and HIV-1 virus [9,13]. Taken together, current data provides evidence that antimicrobial peptides play a vital role in immunity, either by direct antibacterial action or acting on both innate and adaptive branches of immune responses. They are involved in signaling events influencing initiation, development, polarization, magnitude and amplification of the mechanisms that provide an efficient immunity to the host. The focus on understanding the extent and mechanisms of action of cationic host defense peptides has intensified in the last decade, leading to the description of a plethora of novel biological determinants governing their action towards pathogens (Fig. 1). This review describes and integrates advances in understanding the action of antimicrobial peptides during the last years and focuses on the role of these peptides in immunity and their potential in therapeutics, with biophysical and biochemical perspectives. Previous reviews should be consulted for topics that are not extensively described here and for more detailed coverage of areas only briefly mentioned in this review [3,15,19,27,40–48]. 2. Antimicrobial peptide structural diversity 151 2.1.1. Amphipathic a-helical peptides The largest group of the antimicrobial peptides known so far is the linear cationic a-helical peptides where more than 300 different members have been described so far. The most important representatives are cecropins, magainins, cathelicidins and melittin [9,50,51]. These linear peptides have been discovered from numerous sources, including the extracellular fluids of invertebrates, insects, nematodes, teleost fish, frogs and mammalian neutrophils [24]. These peptides are typically 12–37 residues in length, and may have a kink or a central hinge region, as observed for dermaseptins [52] and caerin 1.1 [53]. One of the most studied cationic a-helical peptides belongs to the cecropin family. Cecropins A, B and D are close homologues with 35–40 residues that were firstly isolated from the pupae of the cecropia moth. A mammalian homologue, cecropin P1, was also found in the upper part of the pig small intestine [54]. In the presence of membrane-mimicking environments, Holak et al. have demonstrated that cecropin A adopts an amphipathic helical conformation [55]. Silvestro et al. [56] have further studied the folding of cecropin A in HFP–D2O (HFP – hexafluoro-2-propanol) and 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) monoand multibilayers (MBLs) and it was shown that the folded structure of cecropin A is characterized by a pair of connected helical segments and terminates in short random sequences. The N-terminal a-helix (5–21) has a continuous distribution of basic residues and forms an amphipathic structure that is connected by a flexible hinge region to the more hydrophobic C-terminal helix (24–37) [57]. Another well-characterized group of a-helical membrane-active peptides are magainins, which comprise a family of immunogenic peptides that are expressed in the skin and intestine of frogs. Magainins are 23-residue peptides that exhibit a broad-spectrum antibacterial, antifungal, and tumoricidal activities. Magainin adopts an a-helical secondary-structure upon binding to phospholipid membranes, as determined by several spectroscopic techniques, namely CD (circular dichroism) [58], Raman [59] and solid-state NMR (nuclear magnetic resonance) [60]. Melittin is found in bee venom and exhibits potent broad-spectrum antibacterial activity, but its derivatives are usually highly hemolytic. These 26-residue peptides, like cecropins, are amidated and also appear to structure into two a-helical regions separated by a non-alpha-helical segment at residues 11 and 12, but the polarity is reversed as the N-terminus is hydrophobic and the C-terminus is basic when compared to cecropins [61,62]. An early study by Frey et al. [63] on the orientation of melittin in phospholipid bilayers by polarized attenuated total reflection infrared spectroscopy (ATR-IR) has shown that the peptide folds into an amphipathic a-helix in phospholipid multibilayers of 1,2-dipalmitoylsn-glycero-3-phosphocholine (DPPC), 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC), and a 4:1 mixture of POPC and 1-palmitoyl-2-oleoyl-phosphatidylglycerol (POPG), as well as when bound to supported planar lipid bilayer (SPBs) of POPC:POPG (4:1). Cornut et al. have further demonstrated that melittin is mainly a-helical and presents some disordered domains in DMPC model membranes [64]. 2.1. Classic small peptides The promising applications of AMPs on the treatment of infections and immunomodulation have provided clear evidence of the therapeutical potential of this class of peptides. One of the most intriguing aspects of antimicrobial peptides is the limited sequence homology and the wide range of secondary structures, despite their similar general physical properties. The most prominent structures found are amphipathic a-helices, amphipathic peptides with two to four b-strands and loops and randomized structures [1,11,24,27,48,49]. 2.1.2. Amphipathic b-sheet peptides The b-sheet antimicrobial peptides present a well defined number of b-strands, with relatively few or no helical domains, organized in the common amphipathic pattern. Most peptides are constrained either by disulfide bonds, as in the case of the tachyplesins, protegrins and lactoferricin, or by cyclization of the peptide backbone, as in the case of gramicidin S, polymyxin B or the tyrocidines. The cysteine-containing b-sheet peptides represent a highly diverse group of molecules mainly represented by defensins. These 152 V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 peptides are frequently formed by several antiparallel b-strands, and are stabilized by a series of up to six disulfide bonds. All defensins are cationic and contain 6–8 highly conserved cysteines that pair in three or four disulfide bonds [65]. The structure of the two main defensin subfamilies, a and b-defensins, has been solved by two-dimensional NMR and X-ray crystallography and both consist of a triple-stranded b-sheet with a distinctive defensin fold. Recent data have demonstrated the existence of a common motif that integrates all of the above classes of cysteine-stabilized antimicrobial peptides [65]. This motif, termed the c-core, is composed of two antiparallel b-sheets, with basic residues concentrated along its axis. 2.1.3. Other and specific residue-rich peptides There are many examples of antimicrobial peptides that do not present a specific archetype or motif; instead, they are defined by the predominant presence of a particular aminoacid that imposes particular constraints to their structure [27]. This group of peptides includes those with relatively high amounts of tryptophan (indolicidin) [66], proline and arginine (PR-39), and histidine residues (histatins) [21]. Although some of these peptides are not so recurrent and consequently are less studied, available data shows that the structures of the known peptides with enriched amino acid composition tend to differ from prototypic a-helical or b-sheet structures. Some studies have shown that tryptophan-rich indolicidin [67] seems to adopt a wedge-shaped structure in membranemimetic environments and that some proline–arginine rich peptides, such as PR-39, folds into polyproline helix type-II structures. In particular, the later motifs are specifically bound by SH3 domains and this binding may be relevant for protein–protein interactions [68]. 2.2. Larger antimicrobial proteins and polypeptides Recently, polypeptides and proteins considerably larger than classical short antimicrobial peptides have been shown to have unambiguous antimicrobial activity. Interestingly, some of the smaller peptides have been shown to result from enzymatic proteolytic cleavage from these larger precursors [69]. Peptidoglycan recognition proteins (PGRPs) are an example of this class of proteins. They were first discovered in insects, and comprise a family of variable sized proteins (20–120 kDa) that specifically bind peptidoglycan (the main component of cell wall of bacteria, particularly on Gram-positive bacteria) [70]. In a first approach, insect PGRPs were characterized as pattern recognition receptors (PRR) that instigate downstream immunomodulatory signaling cascades after interaction with immune effector cells through receptor-linked Toll or Imd pathways. Some homologues have already been found in vertebrates, especially in mammals, such as the human PGRP-1, -3, and -4 family and bovine oligosaccharide binding protein (bOBP49) with similar antimicrobial profiles, as described in studies performed with Bacillus (B.) subtilis and Staphylococcus (S.) aureus strains [71]. Lactoferrin is another well known case, being an 80-kDa ironbinding glycoprotein member of the transferrin family that is found in mammalian milk and other fluid secretions such as tears, saliva and seminal fluid [72–74]. It is also found in the secondary granules of polymorphonuclear leukocytes (neutrophils) where it is released during inflammatory responses [72]. This glycoprotein is considered to be a part of the innate immune system. Due to its strategic position on the mucosal surface, lactoferrin represents one of the first defense systems against microorganisms invading the organism. Somehow lactoferrin may support the proliferation, differentiation, and activation of immune system cells and strengthen the immune response [72]. Moreover, lactoferrin affects the growth and proliferation of a variety of infectious agents including both Gram-positive and Gram-negative bacteria, viruses, protozoa, and fungi both by iron deprivation [74] and by a porin-dependent mechanism, as more recently reported for Escherichia coli [75–77]. A number of reports suggest that peptides derived by acidic hydrolysis of lactoferrin have also direct antimicrobial functions [78], for example, lactoferricins (LFcins) that are cleaved from the N-terminus of lactoferrin by pepsin under acidic conditions. It is thus likely that such process occurs naturally in biologically-relevant contexts, such as in the stomach after food ingestion [73,79]. Bovine lactoferricin (LFcin B) and human lactoferricin (LFcin H) are representative cationic peptides constituted by 50 amino acids with one to two disulfide bonds, and some studies have shown that they may present improved antimicrobial properties when compared to the parental protein [78–80]. The effect of LFcin B is initiated by rapid binding to the bacterial and fungal cells in a pHdependent manner. This binding is modulated by divalent cations such as Mg2+ and Ca2+, suggesting the importance of electrostatic interactions in the mechanism of action [78]. LFcin B also causes rapid K+ release and H+ internalization by Candida albicans cells, reduced uptake of proline by E. coli and B. subtilis and reduced uptake of glucose by Trichophyton rubrum [78]. Some human derivatives, such as LFcin H (20–30), induce loss of membrane potential and cell lysis [78], which is also observed for the majority of lactoferrin-derived peptides [69,80–83]. In order to elucidate the mechanism of action, recent studies have demonstrated that many lactoferrin derivatives have a preferential interaction with negatively charged membranes [69,80,81,83,84] and suggest that they perturb the lipid bilayer through membrane permeabilization, possibly by surface insertion followed by membrane thinning [82,85,86]. Other mechanisms have also been described in the literature for other lactoferrin derivatives. Some human lactoferrin derivatives have also been suggested to be capable of binding to unmethylated nucleotides and, therefore, of restraining exacerbated pro-inflammatory events by regulating the activation of immune cells [73]. Moreover, some derivatives have been shown to target organelles in eukaryotic cells, as demonstrated for the N-terminal peptide of human lactoferrin hLF (1–11) [87]. 3. Biological significance of membrane composition as a measure of affinity to antimicrobial peptides The cell membrane is regarded as a defined bilayer of phospholipids that regulate the flux of metabolites between the external environment and the intracellular content. The physicochemical nature of membrane lipids is the crucial basis for the lipid assembly into structural and functional membranes. This physical organization allows the membrane to function as a permeability barrier and limits the occurrence of chemical reactions for the purposes of biochemical energetic efficiency to a particular cellular microenvironment, leading to chemical compartmentalization. The conjugation of lipids with proteins in supramolecular complexes is known to be paramount for many biological processes, namely cellular membrane biosynthesis, cell homeostasis and regulation of membrane fluidity in response to environmental challenges. In the case of pathogens, the membrane is the main target of most antimicrobial peptides. Upon peptide interaction with the membrane, it is known that several factors modulate the peptide’s activity and the extent of its partition to the membrane. The most prominent is the membrane electrical potential, which is dictated by electrostatic interactions between the lipid headgroups and the cationic peptide. The curvature strain of the membrane is mainly dictated by the nature of the lipids. Unsaturated type II lipid phosphatidylethanolamine (PE) naturally promotes a negative 153 V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 higher negative electrical potential ( 130 to 150 mV) and a lack of rigidifying lipids like cholesterol as compared to host cell membranes seem to be key molecular determinants of the selective activity of AMPs towards these organisms [27]. As for other lipids, sphingomyelin (SM) and phosphatidylcholine (PC) are neutrally charged and they are mostly present in eukaryotic cell membranes, as observed in mammalian erythrocytes (Table 1) [4,89]. Therefore, bilayers enriched in the zwitterionic phospholipids PC and SM are regarded as a reliable model of the erythrocyte membrane, to assess the toxicity of AMPs for host mammalian cells [10,11,13,91,95,97]. It should be noted that at odds with bacteria, phosphatidylcholine is also found in fungi (eukaryotic), in variable proportions (Table 1), which may in part explain their lower susceptibility to AMPs as compared to some bacteria [41,98]. Sterols such as cholesterol (mammalian) and ergosterol (fungi), found in eukaryotic but rarely in prokaryotic membranes, are also neutral and they are very important to regulate membrane fluidity, the formation of specific lipid domains and the antimicrobial peptide’s activity (Table 1) [10,12,99]. The condensing and acyl chain ordering effects of cholesterol and ergosterol on phospholipids in their liquid disordered (ld) state is well established, and are assigned to the rigid ring structure of the sterol limiting trans ? gauche isomerization of vicinal phospholipid acyl chains. Since the volume of the membrane’s hydrocarbon region is approximately conserved, a decrease in surface area leads to an increase in membrane thickness. As a result, less peptide is expected to bind to the membrane and the membrane thinning effect induced by AMPs is reduced, leading to a decrease of their potency. This aspect is of major importance since it is believed that toxicity to host cells is partially inhibited by the presence of these lipid components, as demonstrated in many studies. For instance, Raghuraman et al. have shown that the presence of cholesterol inhibits melittin-induced calcein leakage of DOPC lipid vesicles and the extent of inhibition appears to be dependent on the concentration of membrane cholesterol [99]. A similar inhibition by cholesterol has been reported for melittin-induced leakage from PC vesicles [100]. It is interesting to note that PS is also present in the inner leaflet of the membrane of mammalian cells, but does not impart a significant attraction to AMPs since it is not exposed. This may partly explain why AMPs are usually non-cytotoxic despite the presence of this lipid. However, cancer cells contain a small amount of this curvature strain in monolayers due to its inverted cone geometry, in contrast to PC and PG lipids, that do not have that propensity, due to their cylindrical molecular shape. In addition, the hydrophobic interactions between the hydrocarbon acyl chain and the amphipathic peptide (mainly mediated by van der Waals and hydrophobic interactions) can induce lipid packing frustration (e.g. membrane thinning) and have an enormous impact on membrane structure, either by membrane disruption and permeabilization or even induction of non-lamellar phases to relieve the strain stress and the acyl chain packing frustration. This aspect will be described in detail later. A crucial pre-requisite for the development of AMPs or any other therapeutic agent is the selective toxicity, acting only on foreign microorganisms while retaining the structural and functional integrity of host cells. Since they are active over a wide range of pathogens, these peptides have taken advantage of biochemical divergence and evolution inherent to cell membrane composition to act preferentially on pathogens and be harmless to the host. This difference may also account for some of the varying levels of effectiveness that antimicrobial peptides exhibit against different types of cells. Membranes of prokaryotic and eukaryotic cells differ considerably in lipid composition and this aspect is particularly important for the AMPs action, since it is thought to be the basis of specificity of antimicrobial peptides towards the target cell [24,88,89]. This trait is also very important in the definition of more realistic mimetic membranes as a means of deferring important information on the potency of antimicrobial peptides and the possible toxicity for host cells [88,89]. In Gram-negative bacteria, PE is the most abundant lipid, as observed for E. coli (Table 1), [88–93]. This lipid is usually used as a zwitterionic lipid on bacterial model membranes in many biophysical studies [94–96]. In contrast, hydroxylated phospholipids such as phosphatidylglycerol (PG), cardiolipin (CL) and phosphatidylserine (PS) present a negative net charge at physiological pH and are abundant on pathogens membrane (Table 1). These lipids are commonly used in model membranes of bacterial and fungal microorganisms (usually mixed with zwitterionic lipids in a defined stoichiometry) [24,89,91]. As cell membranes composed predominantly of PG, CL, or PS tend to be highly electronegative, this trait partly explains the specificity of cationic antimicrobial peptides towards these organisms [10,11,24,93]. Therefore, the higher proportion of anionic lipids at their membrane surface, a significantly Table 1 Major components of membrane lipid composition of representative species of Gram-negative and Gram-positive bacteria [262,263] (and references therein) and mammalian host cells (erythrocytes) [264]. The lipid content and composition in fungal membranes, as exemplified by the yeast Saccharomyces cerevisiae, is extremely complex and highly dependent on the growth conditions [262] (and references therein). Microorganism Lipid (%) Cardiolipin (CL) a.k.a. diphosphatidylglycerol (DPG) Phosphatidylglycerol (PG) Lysylphosphatidylglycerol (LPG) Phosphatidylethanolamine (PE) Phosphatidylcholine (PC) Sphingomyelin (SM) Phosphatidylserine (PS) Phosphatidic acid (PA) Phosphatidylinositol (PI) Sterol Staphylococcus aureus (Gram-positive bacteria) Escherichia coli (Gram-negative bacteria) OM CM Total lipid 6 12 3 5* 3 – 90 – – – – – – 6 – 82 – – – – – – 19 – 74 – – – <1 – – 57 38 – – – – – – – Saccharomyces cerevisiae (Fungi) ** ** U Ergosterol Erythrocyte Outer leaflet Inner leaflet – – – U 20 40 40 – – U Cholesterol – U 40 20 10 30 – U Present in trace or undetermined amounts. * Lipid composition for whole cells in exponential growth phase. Cardiolipin is a minor component during this growth stage but accumulates towards the stationary phase [265]. ** Major lipids of yeast cell membrane in variable proportions. U 154 V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 lipid (3–9% of the total membrane phospholipids) in the outer leaflet of the membrane, which presumably turn these cells more susceptible to AMPs. This observation is consistent with some studies that demonstrate that AMPs are more toxic to cancer cells than benign cells [18,101,102]. Despite the higher content of PS in the outer leaflet of these cells, this feature only partly explains their increased susceptibility. The membranes of many cancer cells also present a higher expression of O-glycosylated mucines, which creates an additional negative charge on the cancer cell’s surface [18]. Another plausible explanation for the different susceptibilities of normal and cancer cells to AMPs is membrane fluidity. The membrane fluidity of some cancer cell lines is lower than that of normal cells, which may decrease their cytotoxicity. This hypothesis arises from observations that the decrease of membrane fluidity usually shields the membrane from the action of these peptides, as described for the effect of sterols on peptide activity. Indeed, it has been shown that some breast and prostate cancer cell lines exhibited increased levels of cholesterol-rich lipid rafts, which may decrease their susceptibility to killing by AMPs [103]. In addition, tumorigenic cells exhibit a higher cell surface area than normal cells due to a higher number of microvilli, presumably increasing the available membrane surface area to bind a larger amount of peptide [18]. 4. Molecular determinants of antimicrobial peptides Structure–activity relationships (SAR) have become a useful tool to study the molecular determinants governing the biological activity of AMPs towards pathogens. Several comparative analyses have demonstrated that antimicrobial peptides have common motifs that may be used to correlate specific parameters with their biological activity. Several approaches have been used, such as sequence modification methods, minimalist approaches, synthetic combinatorial libraries and template-assisted methods. All these methods are based on the comparative analysis and systematic alteration of peptide’s properties such as sequence, charge, hydrophobicity, amphipathicity, degree of structuring (a-helix, b-sheet and random coil content), size and the balance between the hydrophobic and polar regions (the angles subtended by hydrophobic and polar faces in the structured peptide) in order to obtain representative and valuable information on the relative contribution of each property to the peptide’s biological activity. Therefore, a systematic comparison of common structural parameters of these peptides will be important for a more comprehensive understanding on the way peptides act on killing pathogens and their cytotoxicity [42]. 4.1. Sequence and Structure The evidence collected so far has proven that most AMPs have quite different peptide sequences and despite the absence of evident sequence homology, they tend to adopt similar conformational patterns upon interaction with the membrane [41,42]. One important feature are the capping interactions that are observed at the N-terminus of the peptide, independently of the following residues. Tossi et al. have reported a statistical analysis of residue distribution in the N-terminal region of a-helical AMPs from different sources, showing that glycine in position 1 is highly conserved. This is mainly attributed to the ability to act as a good capping residue and to provide resistance to proteolytic cleavage by aminopeptidases [41,42]. Another important structural aspect is peptide amidation, which is the most common post-translational modification in a wide variety of peptides, such as cecropins, melittin, dermaseptins, PGLa, prophenin and polyphemusin. It is known that amidation prevents the activity of carboxypeptidases on peptides and provides one additional hydrogen bond that may account to the energetics of the folding of a-helical peptides [104]. Lysine and arginine are the most representative amino acids and they are usually concentrated in one stretch of the peptide sequence. This is consistent with the cationic nature of AMPs and accounts for the electrostatic interactions that these residues establish with the negatively charged pathogen membrane, as reported before [41,48]. It is important to realize that a strong electrostatic attraction promoted by these basic residues on the hydrophilic face of the peptide is not per se a prerequisite for interaction, as it also occurs between AMPs and neutral membranes, as shown for cecropin– melittin hybrid peptides [95,105,106] and other peptides [107,108]. Nevertheless, it seems to be important in the initial approach to the membrane as demonstrated by the magnitude of the partition – most AMPs have partition constants that are orders of magnitude higher to negatively charged membranes when compared to zwitterionic ones [95,106,109]. In addition, aromatic residues (mainly tryptophan) are considered to have a pivotal function on the partition, by anchoring the peptide to the membrane, particularly at the headgroup level, as recently reviewed [42,73]. A well-known conserved pattern is their amphipathic conformation, as a result of the polarity and concentration of hydrophobic residues facing one side of the helix and polar residues residing on the other face. This trend is paramount for antimicrobial peptides as the cationic polar domain is particularly important for the initial interaction with the membrane surface whereas the hydrophobic patch will drive the peptide insertion into the hydrocarbon chain membrane core, mostly mediated by hydrophobic and van der Waals interactions. Another important aspect is the degree of structuring, which is best documented for a-helical and b-sheet AMPs, and its impact on peptide’s activity. Many biophysical studies aiming at characterizing the thermodynamics of partition to the membrane have demonstrated that partitioning of polypeptide chains into membranes is usually accompanied by peptide secondary structure formation by the well known partitioning–folding coupling process [110– 113]. This trait will be further explored later in this review. Increasing evidence is showing that structuring (helicity) is a requirement for hemolytic but not antimicrobial activity [114]. Oren et al. [115] have studied diastereomers of melittin and investigated their structure and cytolytic activity towards bacterial and mammalian cells. It was shown that melittin diastereomers lost their a-helical structure, which abrogated their hemolytic activity toward human erythrocytes while retaining their antibacterial activity against Gram-positive and Gram-negative bacteria, as revealed by transmission electron microscopy studies. Similar results were obtained with the diastereomers of the less cytolytic peptide pardaxin [116]. Therefore, this apparent dissociation between peptide helical content and antibacterial activity may indicate that a complex interplay with other molecular determinants must be taken into consideration to elucidate the influence of degree of structuring and the biological activity exhibited by AMPs. Most likely the relative contribution of the positively charged and hydrophobic domains is very important, as suggested by Shai and co-workers [116]. In this study, a series of diastereomeric peptides composed of varying ratios of lysine and leucine residues were investigated. The authors have reported that the highly hydrophobic diastereomers exhibited lytic activity against both negatively charged and zwitterionic phospholipid vesicles and showed both antibacterial and hemolytic activities. In marked contrast, the highly positively charged diastereomers induced leakage only from vesicles of acidic phospholipids and exhibited activity only against bacteria [116]. This particular feature was also highlighted by Dathe et al. for KLAL peptide analogs and a double D-amino acid replacement peptide set [117]. The adsorption and concentration of cationic KLAL pep- V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 tides at the membrane and the membrane-disturbing activity on bilayers of high negative surface charge were found to be dominated by charge interactions, independently of any structural propensity. In contrast, the hydrophobic helix domain was particularly decisive for binding and permeabilization of the membranes of low negative surface charge, as it promotes the insertion of the amphipathic peptide into the hydrophobic core, thus disturbing the lipid arrangement and causing local disruption [117]. 155 are essential for antimicrobial activity. Their architectures range from the a-helical peptides of some amphibians to the cyclic cystine knot structures observed in some plant proteins. Some peptides appear to use metal ions to form cationic salt bridges with negatively charged components of microbial membranes, which in turn favours the interaction with their target organisms. In many cases the role in innate immunity and mechanisms underlying the antimicrobial action of these peptides are not yet fully understood [48,119,120]. 4.2. Charge 4.3. Amphipathicity and hydrophobic moment Most cationic amphipatic peptides present a net positive charge ranging from + 2 to + 9. Upon interaction with the membrane, they are expected to cluster at the lipid-peptide interface, establishing strong electrostatic interactions with the negatively charged phospholipid membranes of pathogens. This represents actually the main driving force for the folding of peptide at the lipid-peptide interface [24]. Indeed, bacterial cytoplasmic membranes (CM) are rich in the acidic phospholipids PG and CL, which are responsible for their overall negative charge (Table 1). The existence of other components, such as the LPS in Gram-negative and teichoic or teichuronic acids in Gram-positive bacteria confers an additional negative charge to the surfaces of these organisms [24]. Similarly, phosphomannans or related constituents, chitin chains and the presence of a layer of b-1,3-glucan also confer a highly negative charge to fungal cell walls. Charge thus seems a critical parameter in antimicrobial peptide’s mechanism of action and the significant difference in membrane chemiosmotic potential and lipid composition between prokaryotes and eukaryotes seems to play a pivotal role on their selective toxicity [15]. However, this association between charge and biological activity is not linear and there are examples of direct, indirect and even inverse relationships between these variables [118]. Giangaspero et al. have performed experiments using a sequence modification approach, by varying several properties on a template peptide, P19 [42]. Studies with several analogs, in which the mean hydrophobicity, amphipathicity and degree of structuring were maintained, have shown that a reduction of charge (ranging from + 6 to + 1) had considerably reduced the peptides’ potency against bacteria. However, it was shown that peptides with increasing cationicity above a threshold displayed increasing hemolytic activity. On the other hand, an analogue with a formal net charge of + 8, presented an improved activity towards yeasts but a reduced activity against bacteria, such as Bacillus megaterium (BM 11 strain) and S. aureus (710 A) [42]. This may be related to a steric impediment for helix formation due to close proximity and repulsive electrostatic interactions between basic residues in the packed structure. Furthermore, this electrical repulsion is likely to decrease the lifetime of the pore, which hinders the membranolytic effect of AMPs. Jiang et al. [14], using the amphipathic a-helical antimicrobial peptide LV13 K as the framework to study the variation of some molecular determinants on the peptide’s activity, have shown a similar pattern. A similar correlation was observed between the increase in the number of positively charged residues and the antibacterial activity, but also here a threshold was found, as a further increase in cationicity resulted in an increased hemolytic activity rather than improved antibacterial activity. More recently, some antimicrobial properties have been described for anionic peptides, such as maximin H5 from amphibians, small anionic peptides rich in glutamic and aspartic acids from sheep, cattle and humans and dermcidin from humans. Anionic antimicrobial peptides were first reported in the early 1980s and they present a similar broad spectrum of action as their cationic counterparts. Their structural characterization has demonstrated that these peptides have a net charge ranging from 1 to 7, and for some of these peptides, post-translational modifications These parameters are crucial for the action of all AMPs since most of them fold into amphipathic structures upon interaction with target membranes. Amphipathicity is conventionally defined as the relative proportion and distribution of hydrophobic and hydrophilic residues or domains within a peptide. One quantitative measure of amphipathicity is the hydrophobic moment, calculated as the vectorial sum of individual amino acid hydrophobicity vectors, normalized to an ideal helix [49,121]. The amphipathic a-helix is the most common conformation, having a periodicity of three to four residues per turn, which is optimal for interaction with biomembranes and folding of monomeric a-helices. In fact, it is well accepted that the amphipathicity of AMPs is essential for their mechanism of action because the positively charged polar face will drive the initial electrostatic attraction to the negatively charged components of the membrane, and then the nonpolar face of the peptides will insert into the membrane through hydrophobic and van der Waals interactions, causing increased permeability and loss of the barrier function [15]. Nevertheless, there is still some controversy regarding the influence of amphipathicity on the biological activity of AMPs. Giangaspero et al. reported that the ability to fold into a helical structure without an amphipathic conformation restricts the potency and range of activity of AMPs [42]. A recent paper by Jiang et al. reports the absence of a linear correlation between amphipathicity and biological activity (both microbiological and hemolytic profiles) on a series of analogs of L-V13 K [14]. In a study with magainin 2 analogs, Wieprecht et al. [122] have demonstrated that peptides with slightly increased hydrophobic moment (while retaining other structural parameters) were considerably more active in permeabilizing vesicles mainly composed of zwitterionic lipids and had no effect on Large Unilamellar Vesicles (LUVs) entirely composed of anionic phospholipids. Nevertheless, both the antibacterial and hemolytic activities of these analogs were enhanced. On this basis, it was proposed that the correlation between permeabilization and binding was related to an increased membrane affinity or to an enhanced permeabilizing efficiency of the membrane-bound peptide. Therefore, the increase of the amphipathicity, quantitatively measured by an increase of the hydrophobic moment, seems to be important for both antibacterial (desirable) and hemolytic activities (undesirable effects). It is interesting to observe that amphipathicity appears to have a negligible effect on peptide–lipid interactions on purely-negatively charged membranes, which may have important consequences on the mechanism of action towards Gram-positive bacteria with no zwitterionic lipids, such as S. aureus. On the other hand, for zwitterionic membranes, where electrostatic interactions are not expected to play the main role, the variation of amphipathicity may have important implications on host cell cytotoxicity [27]. In fact, Kondejewski et al. [123] have provided evidence for the correlation between amphipathicity and hemolytic activity. This group has modulated this parameter using the framework of b-sheet-containing tetradecameric cyclic peptide, GS14, showing that a decrease on amphipathicity resulted in a decrease on the hemolytic profile. 156 V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 Interestingly, Stark et al. [124] have now reported a new category of non-amphipathic hydrophobic antimicrobial peptides to be active against Staphylococcus epidermidis, Corynebacterium xerosis, and E. coli. It was shown that the activity was mostly related to the threshold hydrophobicity required for insertion of the core hydrophobic segment, which suggests that the biological activity of some AMPs is better justified by this threshold value for amphipathicity than by its absolute value. 4.4. Hydrophobicity Peptide hydrophobicity, defined as the percentage of hydrophobic residues within a peptide, is approximately 50% for most antimicrobial peptides [41]. It is an important parameter for peptide’s biological activity as it determines the extent of partition of the peptide into the membrane hydrophobic core [24,41]. The importance of hydrophobicity has been extensively studied, although many authors have demonstrated that, in general, hydrophobicity of a-helical amphipathic peptides appears to have a higher impact on host cell toxicity than on antibacterial action. In fact, highly hydrophobic peptides beyond a threshold are related to higher hemolysis and a remarkable reduction on antimicrobial activity and thus to a decrease in discrimination between host cytotoxicity (hemolytic profile) and antimicrobial activity [8,14]. The relationship between peptide hydrophobicity and membrane permeabilization was examined in an interesting study by Chen et al. [125] resorting to Ala to Leu substitutions on the nonpolar face of the template peptide L-V13 K. Notably, it was shown that the increase in hydrophobicity led to an increased hemolytic activity, as the triple Leu analog (A12L/A20L/A23L) was 62.5-fold more active than L-V13 K against human erythrocytes. A very strong correlation between dimerization and hemolysis was also observed. Interestingly, a relationship between hydrophobicity and antibacterial activity was more difficult to establish. Although the antimicrobial activity of more hydrophobic versions of the parental peptide, A12L and A20L, was improved, with a 2.0- and 3.6-fold increase compared to L-V13 K, the most hydrophobic analog (triple Leu version) had essentially no antimicrobial activity against resistant strains of Pseudomonas (P.) aeruginosa. In another study, Giangaspero et al. [42] have shown that the absence of large aliphatic residues, responsible for the decrease of mean hydrophobicity of the peptide P19(5/U), completely abolished the antimicrobial activity against Gram-negative and Grampositive bacteria. Chou et al. have studied the effect of some parameters, including hydrophobicity, on a series of cationic AMPs of 20 amino acids and it was shown that high hydrophobicity was correlated with increased hemolytic activity, whilst antimicrobial activity was found to be less dependent on this property [126]. Altogether, these studies suggest that optimal activity may be achieved by moderately hydrophobic peptides, as increasing levels of hydrophobicity beyond a threshold level may increase the propensity to dimerize or oligomerize that may cancel the desired antimicrobial activity. From a thermodynamic view point, highly hydrophobic peptides in an aqueous environment are energetically more stable in aggregates than in monomeric form, as this allows shielding of the hydrophobic moieties from the aqueous media. The higher aqueous stability could prevent the partition to the membrane as a higher energetic cost for the partition of peptide aggregates is expected rather than on a monomeric form and therefore the aggregates might display weaker membrane interactions. Moreover, it may become energetically more costly for the peptide units in the aggregate to reorganize and assume the correct folding and orientation upon the partition to form peptide supramolecular assemblies, such as pore-like structures. An interesting interplay between hydrophobicity and amphipathicity was observed by Wieprecht et al. [122]. Although a reduction of the hydrophobicity of magainin 2 amide peptide from 0.036 to 0.096 (L2R11A20 M2a) substantially decreased activity at POPC-rich membranes, the activity could be completely restored, even at this low hydrophobicity, by enhancing the hydrophobic moment (a measure of amphipathicity) from 0.287 to 0.332 (I6R11R14W16 M2a). Conceptually, related observations in systematic studies of the cyclic peptide antibiotic gramicidin S indicate that a balance between peptide hydrophobicity and amphipathicity for therapeutic effectiveness is a key factor for this peptide to be used as a therapeutic agent [127,128]. Another important relationship was established between hydrophobicity and charge. It was reported that charge increase led to a striking increase of hemolytic effect of magainin derivatives and the low hemolytic activity was restored by decrease of hydrophobicity of hydrophobic helix surface [129]. In this case, an increase in net positive charge must enhance accumulation of peptide at lipid head groups (phosphate groups) of erythrocyte membrane whereas a decrease in hydrophobicity may decrease the ability of peptide interaction with the membrane hydrophobic core, thus reestablishing the lower hemolytic profile of the peptide. 4.5. Polar angle Polar angle is a parameter that measures the relative proportion of polar and nonpolar faces when peptides adopt an amphipathic helix conformation. As a reference, an optimal amphipathic a-helix in which one face is mainly constituted by hydrophobic residues and the other one mostly composed of charged or polar residues presents a polar angle of 180o [27]. Therefore, alterations on the predominance or segregation of one type of residue are expected to change this parameter. Many studies have suggested that a smaller polar angle (and therefore a greater nonpolar domain) is related with increased membrane permeabilization [130,131]. The polar angle has been shown to correlate with the overall stability of peptide-induced membrane pores since a study by Uematsu and Matsuzaki [130] has shown a correlation of polar angle on membrane permeabilization and pore formation. Two model peptides with polar angles of 100o and 180o were shown to fold into a typical native a-helical amphipathic peptide and to form pores, but overall data has provided evidence that peptides with smaller polar angles induced greater translocation and pore formation rates [130]. Since a higher stability of pores formed by peptides with larger polar angles is correlated with the formation of larger charged surfaces, and/or more peptide molecules per channel, the ability to disrupt pathogen membranes is necessarily linked to polar angle of AMPs [27]. In summary, these data clearly shows that a subtle interplay between all these parameters plays a pivotal role in defining the range of activity of AMPs and that these features are not independent, since modification of one parameter often leads to compensatory adjustments in others, clearly demonstrating that these interrelationships are key determinants to unravel the mechanism of action and the biological activity of AMPs. 5. Antimicrobial peptide’s mechanisms of action: an overall perspective It is widely accepted that the main mechanism by which antimicrobial peptides exert their action against pathogens is membrane permeabilization. As a consequence, the dissipation of the electrochemical potential, lipid asymmetry and loss of important metabolites and cellular components usually culminate in cell shrinkage and ultimately cell death [27]. Currently, many studies have provided consistent evidence of additional mechanisms, as an increase on membrane permeability alone may not be sufficient V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 to cause cell death. Data supporting this well-accepted concept arises from a distinction between membrane perturbation and cell death as the latter may occur in the absence of significant perturbation in membrane structure. In fact, some authors have reported the existence of mechanisms in which downstream, intracellular sites are regarded as the main targets of antimicrobial peptides [43,132] and receptor-mediated signaling activities of some peptides have also been reported [133]. Direct contact with or affinity for heterotrimeric G proteins has been proposed as a basis for cationic peptide binding to mammalian cells and the process would involve translocation of peptides across the plasma membrane. This mechanism has been referred to when describing the exposure of mast cells to KLA analogue cationic peptides [134]. 5.1. Adsorption and binding to membranes AMPs are thought to be unstructured in solution and to fold into their final amphipathic conformation upon interaction with biological membranes. These peptides must first be attracted to the pathogen cell wall and there is widespread acceptance that the initial mechanism by which antimicrobial peptides target membranes is mediated by electrostatic interaction [4,8,10,13,14,135] (Fig. 2A). This interaction mainly occurs between the cationic charged AMPs and the anionic components of the membrane [8,10– 13,132,136,137], a view supported by the conservation of basic residues within many antimicrobial peptides isolated from different organisms. The precise mechanism by which electrostatic attraction drives peptide–membrane interaction has been extensively studied and many authors emphasize the composition and architecture of 157 Gram-negative and Gram-positive cell wall as the basis for the different susceptibility of pathogens to AMPs. Apart from the anionic phospholipids, the lipid A core of lipopolysaccharides (LPS) of Gram-negative bacteria and the teichoic and teichuronic acids on the surface of Gram-positive bacteria must also play an important role in this context, showing that not only phospholipids are important for peptide-mediated killing action [11,135]. In the case of Gram-negative organisms, Hancock has proposed a mechanism of peptide interaction with membranes termed self-promoted uptake [138], similar to the one described for cationic polymyxin B [139]. According to this model, the initial action of the peptide involves a competitive displacement of LPS-associated divalent cations (Mg2+ and Ca2+), from which peptides destabilize this supramolecular assembly and gain access to both outer and inner membranes [138,139]. For Gram-positive organisms, however, a different approach must be considered as they do not possess LPS or an outer membrane, but instead, a thicker peptidoglycan layer and the negatively charged teichoic and teichuronic acids, which are successfully explored by peptides to interact with the bacteria. A general mechanism by which peptides may permeabilize or rearrange microbial membranes is still under intense debate. However, some aspects are shared by most peptides. In brief, once close to the microbial surface, peptides must cross capsular polysaccharides and other components of the cell wall before they can interact with the outer membrane in Gram-negative bacteria or the cytoplasmic membrane in Gram-positive bacteria [11,49,135,140]. This fact is often disregarded in most mechanistic studies derived from model membranes. The initial interaction with the cell wall usually does not involve any specific receptor, Fig. 2. General mechanism of action of membrane-permeabilizing antimicrobial peptides. After interaction with the membrane through electrostatic interaction with anionic components of the bacterial membrane (such as LPS, CL and PG) (A), peptides fold into a secondary structure (mostly a-helix), concentrate and usually lie parallel at the interface. (B) Reaching the threshold concentration, the peptide adopts an orientation perpendicular to the axis defined by the water/membrane interface or remains on a ‘tilted state’, (C) inserts into the hydrophobic core of the membrane and (D) promote the formation of pore-like structures, which are responsible for the membrane permeabilization. (Adapted with permission from [257]. Copyright 2007 National Academy of Sciences, U.S.A). note: In the original reference, this mechanism is proposed for HIV-Tat, but it presents the general features of the partition and the mechanism of action of AMPs. 158 V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 as enantiomers of lytic peptides, composed solely by D-amino acids, maintain a biological activity comparable to that of their Lcounterparts. The parallelism generally found between antibiotic activity and the ability to alter the permeability of lipid membranes (achiral molecules) rather than interaction with specific receptors also supports this notion [14,41]. However, some studies have provided evidence of some exceptions to this generalization. The initial work of Sahl’s group and others that followed showed that nisin actually acts by a receptor-mediated mechanism, which involves lipid II as the receptor. This is crucial in the initial step of the action of nisin and similar bacteriocins [141,142]. This interaction inhibits peptidoglycan synthesis and forms highly specific pores that result in the depletion of intracellular components. As a result, the combination of highaffinity binding to Lipid II and permeabilization of the plasma membrane potentiates the antibiotic activity and contributes to the high potency exhibited by the peptide in nanomolar minimum inhibitory concentration (MIC) values [141]. Another study has demonstrated that sensitivity of Listeria monocytogenes to the bacteriocin mesentericin Y105 was dependent on ManR, a new sigma(54)-associated activator, and Ell(t)(Man), a new sigma(54)dependent PTS permease of the mannose family. Such PTS permeases are involved in sensitivity of different target strains to mesentericin Y105 and could be potential receptors for the subclass IIa bacteriocins [143]. More recently, Destoumieux-Garzón et al. have demonstrated that the iron-siderophore transporter FhuA is the receptor for the antimicrobial peptide microcin J25 [144]. These studies suggest that receptor-mediated interactions are important for the mechanism of action of some antimicrobial peptides in targeting specific components of the pathogen cell wall, accounting, at least partially, for their mechanism of action. 5.2. Threshold concentration After initial membrane binding, peptides must locally concentrate in order to exert their antimicrobial activity, until they reach a concentration that enables productive action [4,26]. Such feature is commonly described as the threshold concentration concept [4]. At this concentration, peptides begin to rearrange and alter pathogen permeability via current accepted mechanisms, which will be described later. However, a concentration threshold, characteristic for each peptide/membrane composition, seems to be a necessary requisite for their biological activity and it is independent of the subsequent mechanism of action triggered by the peptide [26,106]. Conceptually, the threshold concentration is described as the minimum peptide concentration [or the peptide-to-lipid ratio (P:L) in an experimental perspective] necessary at the target surface to promote its biological effects [26]. At low peptide-to-lipid ratios, the peptide tends to interact and concentrate at the level of the lipid headgroup. At this stage, peptides fold and remain adsorbed parallel to the lipid bilayer (Fig. 2B), probably at the interface of head groups and fatty acyl chains and may already display a significant content in secondary structure [4,105,145,146]. As the P:L ratio increases, peptides begin to orient perpendicular (or in a ’tilted state’ [147]) to the membrane, inserting and partitioning into the hydrophobic core of the bilayer (Fig. 2C). Accordingly, the Huang-Matzusaki-Shai’s two-state model establishes that the threshold value (P:L)⁄ is the peptide concentration at which the energy levels of the S state (the surface adsorbed, parallel state observed at low P:L ratios) and the L state (the pore-forming state, inserted and non-parallel to the surface, above the threshold concentration P:L⁄) are equal. In this perspective, the (P:L)⁄ threshold value is a function of the adsorption binding energy, the elastic constants of the bilayer, and the energy level of the pore state [4]. The most important aspects governing the partition are peptide concentration, propensity to self-assemble or multimerize (both in bulk solution or at the water/membrane interface), the membrane composition, fluidity, and headgroup chemistry and size, transmembrane electric potential and pH, the latter influencing also the charge state of peptides and ionic lipids [4,11,146,148–150]. Threshold concentration and peptide parallel-to-transmembrane surface orientation are also considerably affected by trans-negative membrane potential of many bacterial membranes, since it is the connecting factor between peptides and membrane. At a peptideto-lipid ratio above the threshold value (P:L)⁄, the peptide may promote alterations in conformation, both in-depth membrane localization and association state, as well as indirect changes in bilayer topology, such as pore formation or disintegration (Fig. 2D). Recently, Melo et al. have established that extremely high peptide concentrations are expected at the membrane surface and that those can reach (or be close to) bilayer saturation [26,106]. The saturation (P:L) ratio estimates based on biophysical parameters (partition coefficients, Kp) reproduce observed MIC values, which may imply that such high threshold concentrations are expected at the membrane level in order to produce the biological effects in vivo. Similar considerations had been previously addressed by Tossi et al. [41]. 5.3. Conformational transition One of the most important processes occurring after membrane binding is the rearrangement of the conformation of the peptide at the lipid–water interface, a process to date better documented for a-helical AMPs. Although the precise mechanism by which conformational transition occurs is not fully understood, it is likely that fundamental thermodynamics contributes to such conformational changes. The thermodynamics of the a-helix coil transition of antimicrobial peptides in a membrane environment and the formal implications for the peptide–membrane equilibrium are well characterized and have provided some consistent evidence that have been crucial for the development of thermodynamic models and the clarification of the steps involved in the peptide–lipid interaction mechanism [110–112,151]. It is interesting to observe that this process has evolved to take advantage of thermodynamic equilibrium states by regulating the heights of barriers separating such states. Indeed, such modulation proceeds in a very subtle way since it seems to be adjusted through a refined balance between peptide properties, their interactions with the aqueous media and the membrane environment. The thermodynamics of folding of amphipathic peptides in water and in organic solvents has been extensively investigated [152]. The interaction of peptides with the lipid membrane can be divided into four thermodynamic steps-partitioning, folding, insertion and association, as described by White and Wimley [111]. After electrostatic adsorption and correct orientation according to the plane of binding, the partitioning of the peptide to the membrane and conformational transition are the key stages of the process. Kaiser et al. firstly demonstrated that the partitioning of peptides to membranes can be described by a partitioning– folding coupling process, as the formation of secondary structure makes the partition of the structured peptide energetically less costly [153]. Wimley and White proposed that the formation of peptide bonds upon folding to a structured conformation would reduce the peptide’s partitioning Gibbs energy, contributing to the partitioning pathway [154]. Based on a hydrophobicity scale, it was estimated that the change in Gibbs energy for the process amounts to DGhelix = 0.2 to 0.5 kcal/mol per residue, which is ascribed essentially to hydrogen bond formation. Wieprecht et al. have shown that helix formation was accompanied by a change in Gibbs energy of DGhelix = 0.14 kcal/mol per residue. Calorimetric measurements and non-calorimetric estimates showed that a- V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 helix formation is driven by a negative enthalpy, DGhelix, and opposed by entropy, DShelix. Enthalpy values of DHhelix = 0.7 to 1.3 kcal/mol per residue were reported for helix formation in water, as derived from different systems [110,155–158]. The enthalpy of helix formation was found to be largely independent of the peptide sequence and was mainly attributed to the formation of intra-molecular CO–NH hydrogen bonds [155,156]. Helix formation is opposed by entropy with typical entropy values ranging from DShelix = 2.5 to 4.6 cal/mol.K per residue [110,158]. In M2a and its diastereomers, the entropy change upon interaction with the POPC/POPG (3:1) small unilamellar vesicles (SUVs) was found to be DShelix 1.9 cal/mol.K per residue [110]. This is less negative than reported for the coil–helix transition of peptides in water. The smaller negative entropy change of membrane-induced helix formation may reflect an already decreased conformational freedom of the still unfolded but membrane associated peptide. As a result, helix formation can be considered an important driving force for partitioning–folding coupling arising from the Gibbs energy reduction associated with H-bonding. Another contribution to the overall reduction in Gibbs energy comes from the shielding of peptide bonds into intra-molecular H-bonds in a-helices and b-sheets when the peptide is already inserted on the hydrophobic core of the membrane [159]. This feature was addressed by White and Wimley, that reported that the main source of the peptide stability in a non-polar environment arises from the high energetic cost of breaking H-bonds compared to an aqueous environment – the authors have emphasized that the unfolding of a helix of 20 amino acids within a non-polar environment would require approximately 100 kcal/mol [159]. Although helix formation is indeed important for the conformational transition, the per-residue reduction in Gibbs energy, DGresidue, that drives secondary structure formation in the membrane interface cannot be ascribed to this sole effect, and other interactions must contribute as well, including the effects of folding/assembly entropy, side-chain packing, relative exposure of side-chains to membrane and water, and the depth of membrane penetration of secondary structure units. Perhaps the most striking aspect of the conformational transition is possibly the lack of discrimination between microorganisms, even belonging to different kingdoms. In fact, the simple existence of a bioactive negative surface appears to be a key condition for AMPs to fold from an unfolded peptide into a structured conformation [27]. In comparison, b-sheet AMPs are typically much more ordered in aqueous solution and membrane environments, due to constraints imposed by disulfide bonds and the intrinsic rigid structure of this type of secondary structure as compared to the ahelix. Tam et al. [160] have designed b-sheet cyclic peptides based on the antimicrobial peptide tachyplesin-1 (anti-parallel b-sheet structure connected by a type I b-turn) and they have shown that the design of a highly rigid peptide template may be useful for further enabling to dissociate antimicrobial activity from cytotoxicity, anticipating that conformation transition is also a key step to modulate the biological activity displayed by the peptide. In contrast to a-helical peptides, the common dimerization of b-sheet peptides could also increase antimicrobial activity, by promoting a deeper positioning into the hydrophobic membrane core than would be allowed to a monomer. Besides, the assembly of small peptide aggregates may facilitate the formation of transmembrane pores or channels, as proposed in studies for peptides such as cecropins [161]. Peptide–membrane interactions can also depend on the conformational dynamics of the peptide in solution both at the water–lipid interface or at the target membrane. For instance, the antimicrobial peptide trichogin GA IV acts through a complex equilibrium involving two different peptide species, monomers and small aggregates [5], and it was shown that only the aggregates 159 promote membrane permeability [5,162]. More recently, it was reported that many AMPs, such as temporin B and L, LL-37, plantaricin A, magainin-2, sakacin P and melittin form amyloid–like structures in the presence of acidic phospholipids, which seem important for their mechanism of action [163,164]. Based on the observation of protofibrilliar structures on the folding/aggregation process, the cytotoxic action of these amyloid-forming peptides resembles the supramolecular protein–lipid amyloid-like fibers formed upon binding to negatively charged phospholipid-containing membranes, as demonstrated for Ab, prion, a-synuclein, and IAPP [163]. Within this framework, it was demonstrated that these AMPs form fibrilliar protofilaments at the membrane, preceding the formation of inert and non-toxic mature amyloid fibers, after which the membrane permeability is compromised, demonstrating that conformational dynamics (aggregation/protofilament formation) is indeed important for the free energy landscape and specificity of action exhibited by some AMPs. 5.4. Peptide insertion and membrane permeability 5.4.1. Membranolytic activity Many authors have explored this feature using different biochemical and biophysical approaches, such as NMR, Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction. As a result, several models have been proposed to explain how, following initial attachment, antibacterial peptides insert into the bacterial membrane to form transmembrane pores (membrane permeabilization and lysis) [13,165]. On the other hand, other processes by which transient topological alterations on membrane structure occur have been recently described as a means to gain access to intracellular targets [5,9,26,48,132,166]. Here, we provide a comprehensive perspective of the current knowledge on the mechanism of action of AMPs and incorporate some novel aspects that have been unraveled in the past few years. Several mechanisms of action for AMPs have been recently reported, and include membrane permeabilization through the formation of stable pores (either barrel-stave or toroidal pore models), membrane thinning (molecular electroporation or sinking rafts models) or micellization on a detergent-like way (carpet model). Following access to the intracellular space, the sequential leakage of ions and other metabolites, loss of cytoplasmic components, dissipation of electrochemical potentials and ultimately cell death may explain the peptide’s lytic action over pathogens. In the ‘barrel-stave model’, peptide helices form a bundle in the membrane with a central lumen, much like a barrel composed of helical peptides as the staves. This type of transmembrane pore is unique and is induced by quite hydrophobic peptides such as peptaibols alamethicin and zervamicin [165,167,168]. In this mechanism, the hydrophobic domains of a-helical or b-sheet peptides face inwards and interact with acyl chains of the membrane core, whereas the hydrophilic face forms the pore lining (Fig. 3A). The initial step in barrel-stave pore formation involves the initial binding and attachment process, which seems to be accomplished by monomeric but not aggregated forms. After binding, the peptide may undergo a conformational phase transition, forcing polar-phospholipid head groups aside to induce localized membrane thinning. At this point, the hydrophobic portion of the peptide is inserted into the membrane to an extent corresponding to the hydrophobic part of the membrane’s outer leaflet since most peptides do not present sufficient length to transverse all membrane. When the bound peptide reaches the threshold concentration, peptide monomers self-aggregate and insert deeper into the hydrophobic membrane core [15]. Continuous association of peptide monomers may result in further expansion of the membrane pore and upon phospholipid translocation or relaxation of the pore, peptides are transported to the inner membrane leaflet due to the 160 V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 Fig. 3. Antimicrobial peptide mechanisms of action. In this figure, various models are shown, illustrating advances in proposed mechanisms of antimicrobial peptide action. (A) In the barrel-stave model, the peptides span the membrane and form a pore with the hydrophilic portion lining the pore. (B) The carpet model is characterized by the spanning of the membrane by the peptide followed by a detergent-like action that disrupts the membrane structure. (C) The toroidal model differs from the barrel–stave mechanism as the hydrophilic portion of the peptide (in its amphipathic conformation) is associated with the lipid headgroup. (D) In the molecular electroporation model, the interaction of the cationic peptide with the pathogen membrane promotes an electrical potential difference across the membrane. When this potential reaches 0.2 V, a pore is believed to be created by molecular electroporation. (E) The sinking raft mechanism proposes a mass imbalance between the two leaflets of the membrane induced by the peptide. By creating a curvature gradient along the membrane and by self-association, peptides sink into the membrane and form transient pores that are thought to promote a transitory increase on membrane’s permeability and leakage of intracellular contents. After membrane relaxation, peptides will reside on both leaflets of the membrane. (Adapted with permission from [73]. Copyright 2006 Elsevier, UK). concentration gradient of surface-bound peptide, the tension exerted by peptides locally as well as the trans-negative electrochemical potential [169]. In the ’carpet model’, peptides adsorb and span the bilayer surface. This model explains the activity of antimicrobial peptides such as ovispirin [170], dermaseptin natural analogues, cecropins, caerin 1.1, trichogin GA IV [169] and some magainins [171]. Peptides are electrostatically bound to the anionic phospholipid head groups at numerous points, covering the surface of the membrane in a carpet-like manner (Fig. 3B). At the critical threshold concentration, the peptides might form toroidal transient holes in the membrane, allowing additional peptides to access the membrane, from which the membrane disintegrates and forms micelles after disruption of the bilayer curvature [27]. From this perspective, membrane structure disruption occurs in a dispersive-like manner rather than channel formation, and peptides do not necessarily insert into the hydrophobic membrane core, as observed for cecropin P1 [172]. In the ’toroidal-pore mechanism’, antimicrobial peptides partition into the membrane and continuously induce a bending in membrane leaflets through the pore so that the water core is lined by both the inserted peptides and the lipid head groups [27]. The constitution of a toroidal pore implies that the polar faces of the peptides associate with the polar headgroups of the lipids due to bending and therefore, the lipids tilt from the lamellar normal and connect the two leaflets of the membrane, forming a continuous bend from the top to the bottom in a toroidal pore manner (Fig. 3C) [27]. This creates an unfavorable elastic tension that may culminate in the formation of transient defects and ultimately pore disintegration. Yeaman et al. suggest that some peptides may cross through the membrane and act on intracellular targets [27]. Molecular dynamics simulations have also shown that peptide aggregation, either prior or after binding to the membrane surface, seems to be a prerequisite to pore formation although a stable secondary structure is not required [173]. This type of transmembrane pore is induced by some magainins, protegrins and melittin. The toroidal model differs from the barrel-stave model as the peptides are always associated with the lipid head groups even when they are perpendicularly inserted in the lipid bilayer V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 (Fig. 3). Indeed, this seems to be of major importance since the presence of several peptide monomers in the pore would result in a thermodynamically unfavorable Coulomb energy due to an excessive density of charges lining the pore. Therefore, the association with the anionic headgroup of the lipids partly cancels the positive net charge of peptides and favors the peptide aggregation process leading to pore formation. Both the toroidal and carpet models predict that killing activity of antimicrobial peptides occurs with concomitant dissipation of the membrane potential, due to the collapse in membrane integrity. However, the peptides elicit different biological responses and a large set of studies have provided evidence that ion channels, transmembrane pores and extensive membrane rupture followed by leakage of metabolites and intracellular content in general do not proceed through three completely different modes of action, but instead are a continuous gradation between them. This concept corroborates the observation that the formation of peptide induced ultrastructural lesions lags behind the loss of cell viability. Initially, the transmembrane potential and pH gradient are impaired, the osmotic regulation is affected and membrane integrity is then compromised in a concentration-dependent manner. For instance, cecropin A dissipates ion gradients in synthetic lipid vesicles at a concentration much lower than that required to release encapsulated calcein, indicating that the activity of cecropin A at low concentrations is primarily due to the dissipation of transmembrane electrochemical ion gradients rather than membrane permeabilization by pore formation [174]. Interestingly, cecropin A dissipated ion gradients even in the absence of anionic lipids, although their presence dramatically increased peptide binding and moderately increased the release of calcein. In Gram-negative bacteria, cecropin A was considerably bactericidal at similar concentrations which promoted ion conductance changes, but much higher concentrations were required to cause the release of cytoplasmic contents. 5.4.2. Non-membranolytic activity Although permeabilization of the cell membrane seems fundamental to the antimicrobial effect of AMPs, several studies indicate that permeabilization alone may not be enough to explain antimicrobial activity [43]. In fact, antimicrobial peptides may also affect cell membrane topology by creating transient defects on its structure or modulate particular core metabolic pathways after crossing the membrane and gaining access to intracellular targets, such as DNA, RNA, enzymes and even organelles such as mitochondria. 5.4.2.1. Aggregate channel model. Wu et al. have demonstrated that individual peptides present significant differences on their ability to depolarize the cytoplasmic membrane potential of E. coli, such as the loop peptide bactenecin and the a-helical peptide CP26 being unable to cause depolarization at the MIC [175]. This implies that membrane depolarization by itself is not necessarily the crucial event in the killing of microorganisms by these peptides. On this basis, the aggregate channel model was proposed [15]. After binding to the phospholipid head groups, the peptides insert into the membrane and then cluster into unstructured aggregates that cover the membrane. These aggregates are proposed to be associated to water molecules providing channels for the leakage of ions and possibly larger molecules through the membrane. This model essentially differs from the other models as only short-lived transmembrane clusters of an undefined nature are formed, which allow the peptides to transiently cross the membrane without causing significant membrane depolarization and membrane structure disruption. Once inside, the peptides may home to their intracellular targets to exert their lethal activities by acting on polyanions, such as DNA or RNA [15]. 161 5.4.2.2. Molecular electroporation model. Another mechanism that has been proposed for some antimicrobial peptides is the molecular electroporation model (Fig. 3D). This mechanism establishes that the formation of pores in membranes occurs under the influence of an external electric field. When short pulses are used, a voltage of about 1 V across the membrane is required for pore formation. The threshold falls to about 0.2 V for electric fields applied over long time periods (0.1 ms) [176]. Molecular electroporation only occurs when the peptides present a sufficient charge density to generate an electric field and is triggered during the period of time in which the electrostatic potential is at least 0.2 V. This model has been proposed to explain membrane pore formation by annexin V [177]. Pore sizes of 2–4 nm diameter have been reported by conventional electroporation and by at least two other cationic peptides, polymyxin B and melittin [176]. These membrane pores present comparable sizes to the NK-lysin–membrane interface area with a potential 0.2 V greater [176,178]. This mechanism is particularly important to describe the action of those peptides that present antimicrobial activity without apparent formation of transmembrane pores, providing new insight for the means by which peptides increase membrane permeability without necessarily causing its disruption. 5.4.2.3. Sinking-raft model. More recently, a novel mechanism has been reported according to which, the biological activity displayed by some AMPs is a result of imbalance of mass ratio for preference of binding to a particular lipid domain, locally producing a mass disproportion that directs the peptide translocation through increase in local membrane curvature (Fig. 3E). This mechanism, commonly named the sinking raft model, is responsible for the formation of transient pores after the dissipation of the peptide-induced membrane leaflet mass imbalance [179,180]. Pokorny et al. [181] have reported that d-Lysin, an a-helical amphipathic peptide, binds more efficiently to the outer leaflet of the mammalian cell membrane, which are enriched in sphingomyelin, cholesterol and unsaturated phosphatidylcholine. Mixtures including these lipids have been shown to exhibit phase segregation between liquid-disordered (ld) and liquid-ordered (lo) domains, the last one particularly rich in sphingomyelin and cholesterol (lipid rafts). In such systems, Pokorny et al. established that the peptide preferentially binds to the ld domains, producing a local concentration of dLysin and enhancing peptide aggregation in these domains, which, in turn, creates the mass imbalance [181]. Therefore, the curvature strain is relieved as the peptides bind to membranes, sink into the bilayer, and translocate to the cytoplasmatic leaflet of the membrane in a process that perturbs the membrane and causes the efflux of intracellular metabolites [180]. In this case, the equilibrium across the membrane is attained after peptide translocation between the two leaflets, which concomitantly ends the transient ion leakage and small metabolites release [180]. The same model has also been proposed for polyphemusin [182]. 5.4.2.4. Peptide-induced lipid segregation mechanism. Some peptides have been shown to produce significant membrane perturbation by formation of specific lipid–peptide domains, lateral phase segregation of zwitterionic from anionic phospholipids and even the induction of non-lamellar phases at physiologically-relevant conditions. These observations lead to the proposal of new models for the mechanism of action of some AMPs. One of the most intriguing models arises from current observations of peptide-induced lipid segregation of anionic components from zwitterionic lipids [95,96,137] (Fig. 4) and even de-mixing of anionic phospholipids in Gram-positive model membranes [183]. Such feature was observed by Arouri et al. in a study on the influence of linear and cyclic arginine- and tryptophan-rich antimicrobial peptide analogues (RRWWRF) on the thermotropic 162 V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 Fig. 4. Representation of lipid rearrangement upon binding of cationic antimicrobial peptides (blue) according to the lateral lipid segregation mechanism. Clustering of anionic lipids (red) into separate peptide–lipid domains and segregation of zwitterionic lipids (yellow) occurs as a consequence of binding of the antimicrobial peptide, causing a rearrangement of lipids on the membrane with possible significant consequences on cell viability and survival. phase transitions of lipid membranes [96]. It was shown that the presence of both peptides led to the appearance of two peaks in the DSC curves, assigned to a DPPG peptide-enriched and a DPPE-enriched domain, indicating thus induced de-mixing in DPPG/DPPE bilayers. Based on these results, this group proposed that peptide induced lipid segregation in PG/PE membranes could be a further specific effect of the antimicrobial peptides operating only on bacterial membranes, as they contain appreciable amounts of these two lipids (Table 1). In addition, membranes composed of PG and PE do not mix homogeneously, which further favours lipid demixing [88]. Epand et al. have also recently proposed that antimicrobial peptides with a high positive net charge, conformational flexibility and sufficient hydrophobicity facilitate preferential interaction with anionic lipids and the promotion of lipid lateral segregation [137]. It was also hypothesized that peptides that fulfill these requirements are more active towards bacteria composed of zwitterionic and anionic lipids, due to the lipid segregation mechanism proposed. This is supported by a study by Epand et al. [93] using OAK C12 K-7a8, which shows that Bacillus cereus (a Gram-positive bacterium with a high content of the zwitterionic PE) has a considerably lower MIC value than the one found for Gram-positive bacteria whose membrane is mainly constituted by anionic lipids, as observed on S. aureus. In this later species, the much lower percentage of zwitterionic lipids [137], implies that it must be able to counteract more effectively the segregation mechanism because there is no significant proportion of neutral lipids to segregate from the anionic ones. Additionally, the presence of higher content of negatively-charged lipids, such as cardiolipin (CL) and PG, is likely to require a higher peptide concentration to neutralize the mem- brane. These observations are consistent with the higher MIC usually observed for these types of bacteria [137]. A similar trend was observed for a series of monomethylated derivatives of cecropin A-mellitin hybrid peptide CA(1–7)M(2–9). Calorimetric studies [95] have provided evidence of a lipid lateral segregation mechanism in DMPC:DMPG (3:1) and POPE/POPG (3:1) bacterial lipid model systems, and microbiological studies have demonstrated higher MIC50 values for S. aureus (mostly anionic phospholipids) than for the Gram-negative bacterium Acinetobacter baumannii (which has zwitterionic lipids in its membrane composition) [184]. Further evidence was provided by other studies performed by Epand et al. [185] on LL-37 derivatives which indicated that the peptide fragments with the ability to induce lipid phase segregation of anionic lipids away from zwitterionic lipids were active/selective toward bacteria containing zwitterionic and anionic lipids in their cytoplasmic membranes, but presented no activity towards species with only anionic lipids. Overall, these studies suggest that some antimicrobial peptides are able to induce lipid segregation on species with membranes composed of zwitterionic and anionic lipids and that pathogens with such membrane constitution appear to be more susceptible than organisms mostly composed of anionic lipids. Some biophysical and biochemical implications of the action of these peptides on the bacteria based on this mechanism will be addressed now. The occurrence of lateral lipid segregation promoted by the peptides may create membrane line defects that are responsible for the increased permeability of the membrane. This is in agreement with the results for SYTOX leakage of liposomes reported by Fernández-Reyes et al. for the monotrimethylated derivatives of CA(1–7)M(2–9) reported above [184]. In a biological context, this would cause primarily the loss of the permeability barrier property, which would lead to unregulated diffusion of ions and metabolites. The effect of lipid segregation was already reported by den Hertog et al. in Candida albicans where LL-37 and its truncated variants were able to induce this phenomenon on the cytoplasmic membrane [186]. Additionally, the perturbation of the membrane by a coupled mechanism involving pore formation and lipid segregation would amplify this effect, further contributing to the increase of the membrane permeability and impairment of the cell metabolism, as proposed by Teixeira et al. for the CA(1–7)M(2–9) peptide on the Gram-negative mimicking membrane system, POPE:POPG (3:1) [95]. However, the biophysical alterations to the membrane due to lipid segregation can have other implications, namely in terms of curvature strain whose importance in bacterial metabolic processes has been extensively reviewed. A study by Iwamoto et al. [187] has revealed that phosphatidylethanolamine on the yeast plasma membrane is implicated in cell polarity, since the treatment of Saccharomyces cerevisiae cells with a biotinylated probe, which specifically binds to PE, resulted in aberrant F-actin accumulation, implying that limited surface exposure of PE is involved in the polarized organization of the actin cytoskeleton. Similar PErich domains were observed in the septal regions of the cells of many Bacillus species [188]. The same lipid is also involved in cytokinesis as shown by studies with a mutant cell line with a specific decrease in the cellular PE level. The cultures present limited cell growth because the contractile ring remains in the cleavage furrow, promoting the arrest of the cell in intermediate states of cell division [189]. Recent studies have also highlighted the importance of monolayer and bilayer curvature for the budding and fission of biological membranes [190]. Overall it is likely that the activity of antimicrobial peptides may affect the curvature strain and modulate similar relevant biological processes. It would introduce a high destabilization of the membrane beyond the capacity of the bacteria to rearrange and accommodate this change in its organization [96] with relevant V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 consequences on biological processes dependent on the curvature strain of the membrane, such as cell division and sporulation, as stated before [191]. One particular property of lipids that prevails is their phase behavior. In cells, lipids can adopt various fluid and liquid-ordered phases, which are characterized by a different spatial arrangement and motional freedom of each lipid with respect to its neighbors. Biophysical approaches have defined the principles of coexistence of two fluid phases (with different physical characteristics within a single membrane plane) that are delimited by a phase boundary, and the consequences on membrane organization have been pointed out, such as lateral phase segregation or domain formation [90]. Indeed, membrane lipids can occur in various phases depending on their composition, structure and environment [88]. These phases have specific properties that determine the orientation, packing and mobility of membrane lipids and proteins and the interactions between them, which in turn have an important impact on many biological processes. In this context, apart from lateral lipid segregation, it is likely that the peptide-mediated phase segregation by the formation of PE- and PG-enriched domains (with different physical states) would have an enormous impact on membrane fluidity since it would diminish the lipids’ diffusion rate, the lateral movement of proteins and particularly the required fluidity of the membrane for cell viability [192]. Recently, the existence of specific ’functional’ membrane domains has been extensively studied. These domains recruit both lipids and proteins from the cytosol that assemble into specialized microdomains with important functions on the cell dynamics and signaling. The best example are the lipid rafts, which comprise small, dynamic assemblies with high concentrations of cholesterol, sphingolipids and saturated phospholipids that create a local area of increased order and, potentially, bilayer thickness, with important implications on membrane fluidity and organization. Certain proteins seem to be localized preferentially in raft lipid regions, and it has been suggested this may contribute towards the clustering of specific proteins for processes such as signal transduction and even pathogenesis, such as Alzheimer’s disease [193]. In E. coli, similar ’functional’ membrane domains are proposed to have an important role in cell cycle events, such as timing of DNA replication and as space marker for cell division, as demonstrated by Norris et al. [194], as well as in chromosome segregation [195]. Therefore, the functionality of these domains is likely to be modulated by AMPs. From this perspective, an alternative mechanism would involve the loss of the physiological role of these natural-occurring domains due to phase boundary defects resulting from the peptide-induced reorganization and de-mixing of lipids in the membrane. The occurrence of lipid segregation could promote the dissolution of these peptide–lipid complexes and turn them non-functional for cells. 5.4.2.5. ’Leaky-slit’ mechanism. The formation of specific peptide–lipid interactions has been recently reported as well. Zhao et al. have provided evidence of an additional mechanism of action based on a possible connection between fibril formation and the toxicity displayed by some AMPs (Figs. 5 and 6), in line with the mechanism that has been described for lytic bacterial toxins, such as cytolysin [196] and other cytotoxic proteins [197]. According to this model, the lipid-bound peptides adopt a linear, amphipathic array with the hydrophobic side facing the lipid bilayer to promote membrane insertion. In this case, the toxicity would be caused by the hydrophilic domain of these aggregates, which allows self-association of the peptides by hydrogen bonding in the target cell membrane, leading to initial aggregation and formation of fibrilliar and toxic oligomers (Fig. 5). As a consequence, lipids would be forced to adopt a highly positive curvature and transient toxic oligomers (‘leaky slit’) would increase the mem- 163 Fig. 5. Illustration of the ‘leaky slit’ model as a possible mechanism of action of antimicrobial peptides. The peptide adopting an amphipathic alpha-helix is depicted in blue and the lipid headgroups and chains are shown in bright grey and black, respectively. (Adapted with permission from [164]. Copyright 2006 Elsevier, UK). Fig. 6. Microscopy images of fibrilliar structures formed upon interaction of plantaricin (plA) with DOPC/brain PS-containing liposomes. (A) Fibers stained with Congo red and viewed under a polarizing microscope. (B) Fluorescence microscopy image of fibers incubated in the presence the fluorescent phospholipid analogue NBD-PG, which was additionally included in the PC/PS liposomes. The analogous formation of fibrous aggregates consistent with supramolecular protein–lipid fibers formed by several other cytotoxic proteins and peptides are highlighted. The observed fiber formation promoted by the presence of anionic phospholipids is thought to be directly related to its cytotoxicity, similarly to the toxicity of fibrils formed by the paradigm of amyloid-forming peptides. (Adapted with permission from [164]. Copyright 2006 Elsevier, UK). brane permeability to metabolites. At the end, the oligomers would convert into inert amyloid-like fibers, detoxifying the peptides. The authors have stated that the requirements to promote these effects on target cells are fibrilliar organization and amphipathic nature of the fibril, spanning the bilayer. Factors like lipid composition and the molecular determinants of the peptide, mainly conformational flexibility, amphipathic nature, and propensity to fold into amyloid-like structures are likely to greatly influence the peptide activity at the membrane level. In fact, plantaricin A and other similar peptides tend to fold into amyloid-like fibers mostly in the 164 V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 presence of negatively charged liposomes causing vesicle aggregation and leakage of vesicle content in membranes containing both zwitterionic and anionic lipids (Fig. 6) [164]. In addition, the fibril could vary in length and could be constituted by a-helical as well as b-sheet structures and the peptides may be long enough to span the bilayer or require dimer formation [97]. These fibrils could further incorporate lipids by orienting the lipid head groups to face the hydrophilic surface of the fiber, while the acyl chains would protrude from the hydrophobic surface and extend into the bilayer, as described by the ‘toroidal-pore model’. Accordingly, the ‘leaky slit’ model readily complies with the profound toxicity of fibrils. The authors have cautiously referred that other related mechanisms, both ‘carpet’ and the ‘toroidal pore’ models could not be excluded to explain the interaction of these peptides. Alternatively, it was suggested that the ‘carpet’ and ‘toroidal pore’ models and the proposed ‘leaky slit’ mechanism, could somehow overlap and contribute to membrane perturbation by antimicrobial peptides. Although the ‘leaky slit’ mechanism could be regarded as a refinement of these two models, this model provides new aspects, in particular the importance of the amphipathic conformation and the fibrilliar nature exhibited by some AMPs for their toxicity towards target cells. In addition, the model can also predict how these fibrilliar protofilaments can induce positive curvature in membranes, in a different manner to what is proposed on the ‘toroidal pore’ model and account for their action on the membrane. Even though this model provides new insights on bridging antimicrobial peptide biological activity and the cytotoxicity shown by other amyloid-forming peptides, it is important to emphasize that more detailed molecular aspects of the process of amyloid-like fiber formation by AMPs and the importance of structural features of peptides and membrane composition at different stages of the aggregation/folding landscape are needed in order to obtain a more comprehensive picture of the membrane association and cytotoxicity displayed by these peptides. This would contribute to understand elusive aspects of the very striking degree of similarity between the action of AMPs and the disease-associated amyloid forming peptides on membranes. 5.4.2.6. Peptide-mediated non-lamellar phase formation mechanism. An exciting research field has emerged from studies showing a possible correlation between non-lamellar phase formation and the activity of cationic amphipathic peptides. Membrane lipids can self-assemble into numerous different phases in aqueous solution, including micellar, lamellar, hexagonal and cubic phases (Figs. 7 and 8). The tendency to acquire a particular membrane organization arises from the well recognized shape-structure concept, as reviewed by Haney et al. [182]. Mixtures of lipids that present similar lipid headgroup and acyl chain cross sections tend to form a planar bilayer structure, such as PG + PC. Lipids that are cone-shaped like PE (due to a higher acyl chain volume-to-lipid headgroup proportion) tend to favor negative mean curvature so that the polar/apolar interface curves towards the polar region. These lipids prefer inverted micelles and inverted hexagonal lipid phases or regions with high negative membrane curvature strain. Most biologically-relevant lipids are mostly bilayer-forming molecules, but can form either lamellar or non-lamellar phases under specific conditions [182]. However, the phase properties of biological membranes are far more complex, since the biological membranes are constituted by a wide variety and assembly of lipids and proteins that overall influence the membrane dynamics. In biological systems, the membrane is mostly depicted as a flat structure in the lamellar phase. Due to environmental factors or lipid lateral stress induced by proteins or other lipids, the membrane may alter its structure to form a phase possessing curved interfaces with conformationally preferred curvature strain and thickness, such as hexagonal, cubic or even micellar phases. According to Kirk et al., the total free energy of the lipid–water assembly is mainly determined by the conjugation of four factors, namely the membrane curvature elasticity, gC; the packing of the hydrocarbon chains, gP; the hydration force; and the electrostatic contribution [198]. Membrane dynamics derives both from inherent factors (such as the length, packing density and geometry of acyl chains of component lipids, the size of lipid headgroup and the resulting spontaneous curvature profile) [13,93] as well as from all external forces that modulate membrane shape and structure [88,89,93,146]. Therefore, it is important to understand how membrane properties governing membrane dynamics are affected by changes induced by the interaction with AMPs. In the absence of stress factors, the stored curvature elastic energy translates into the bilayer expanding laterally and across the headgroup area, increasing above its preferred value in order to release the lateral stress and lipid packing frustration and adopting the thermodynamic most favorable membrane organization. However, this effect can only be tolerated up to a threshold as small alterations of membrane conformation may involve a very high energetic cost due to the exposure of lipid acyl chains to water (commonly known as the hydrophobic effect). Upon stress, transition to an inverse phase may occur. In this case, the interface can bend inwards the aqueous compartment, allowing the headgroup area to decrease and the chains to expose to the solvent hence, releasing the stored lateral stress. A similar feature was observed for some integral membrane proteins, which may release stored curvature elastic stress locally during insertion into the bilayer by allowing the chains to dislocate more and forcing the head groups together, making the peptide–lipid assembly thermodynamically more stable. Fig. 7. Illustration of the lamellar (La) and the hexagonal phases (HI, hexagonal phase and HII, inverted hexagonal phase) of the lipid membrane. A schematic lipid molecule is depicted in the figure, with the hydrophilic headgroup represented in red and the hydrophobic alkyl chain in grey. (Adapted from reference [258]. Copyright 2009 PMC Biophysics). V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 165 Fig. 8. Schematic structures of lipid cubic phases. The inverse bicontinuous cubic phases Ia3d, Pn3m and Im3m are shown, together with the micellar cubic phases Fd3m and Pm3n. The two types of inverse micelles in Fd3m (open and grey spheres) are indicated on each site of the cubic lattice, along with their polyhedral shapes. The current models proposed for the cubic phase with space group Pm3n by Tardieu and Luzzati (A) [259], Fontell et al. (B) [260], and Charvolin and Sadoc (C) [261] are also shown. Adapted from references [209] and [258]. The major importance of this lipid polymorphism in some biological processes has been demonstrated. However, its importance was underestimated for many years. Some examples of the importance of the formation of non-lamellar phases, in particular, cubic phases, have been reported in recent years and its biological relevance assessed (Fig. 8) [182,199,200]. Luzzati et al. [201] have mentioned that phase Q227 (Fd3m) may have important biological implications. Indeed, phase Q227 is observed with some of the most common lipid components of biological membranes (PC and PE) in conjugation with some intermediates derived from enzymatic degradation [fatty acid (FA) and diacylglycerol (DAG)]. Evidence has shown that enzymatic degradation induces the formation of lipid patches and the local structures appear to adopt phases Q224 (Pn3m) and Q227 (Fd3m). These metabolites (FA and DAG) destabilize the membrane and alter its permeability, turning it leakier and more susceptible to a lipolytic agent. However, when FA and DAG are directly incorporated into the membrane, this leakage stops due to alteration in the lipid topology [201]. Another example arises from studies performed on Archaebacteria, a particular group of organisms with challenging physiology that thrive in extreme conditions of pH, temperature, and ionic strength. A prominent difference between archaebacteria and other organisms resides in the chemical structure of the lipid molecules that have considerable implications on the colonization of such adverse environments. This aspect is paramount if one considers the molecular adaptations that these bacteria had to undergo in order to survive in such an extreme habitat (in excess salinity and at temperature above 80 °C). One important example of the biological relevance of such phases arises from a study by Deatherage et al. [202]. The plasma membrane of Sulfolobus solfataricus is supported by a protein layer, the S-layer, that remarkably exhibits a precise epitaxial relationship with the maximally hydrated Q224 phase of the polar lipid extract, suggesting that the plasma membrane is tightly folded in vivo according to the symmetry of phase Q224. The result is a system of two mutually intertwined and unconnected 3D water channels that resembles the cubic membranes. The biological implications of such a complex interconnected channel network were assessed by Luzzati et al. [201]. One end of the channels of one of the two networks communicates with the cytoplasm; the other end is closed by the S-layer. The channels of the other network communicate with the extracellular medium via the channels of the S-layer and must end on the cytoplasmic side. In this structure, the hydrocarbon layer is topologically equivalent to a lipid bilayer constituting a 2D septum separating the cytoplasm from the extracellular medium. The total area of this septum, however, is highly variable according to the thickness of the cubic crystallite. According to Luzzati et al. this model may have important biological implications: by a proper poising of passive ionic permeability across the bilayer and ion diffusion along the water channels, a steady state may be established, leading to a smooth pH gradient along the two channel systems. As a result, the local pH difference across the hydrocarbon layer, against which the protons must be actively pumped, may be much smaller than the difference between the cytoplasmic and the external pH (6.5 and 2, respectively). Furthermore, the unusual metastability of the cubic phase may also be biologically relevant, and it appears to be associated with the bipolar lipid molecular nature (archaeal tetraether bipolar lipids). These molecular nature bear two headgroups, each anchored at opposite polar/apolar interfaces: any phase transition involving a migration of lipid molecules is obstructed by the presence of two independent diffusion processes, therefore turning the transition more difficult to occur. The presence of bipolar lipids, likely to preserve the high-temperature native organization of the membrane at low temperature, is believed to be the evolutionary response taken by these bacteria, and other thermophilic archaebacteria, to this challenge. Many authors have established that the physical properties of the membrane are modulated by proteins and peptides and they may induce non-lamellar phases by altering the curvature strain, 166 V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 the topology of the membrane and the spatial arrangement of lipids and water molecules. On the other hand, they may also act by changing the physical properties of the lamellar phase since it is known that the spatial disposition and packing of lipids in the membrane is closely controlled by the cell, in order to maintain the flat, mostly planar morphology of the membrane [149,182,199,203]. Therefore, how can antimicrobial peptides modulate the membrane dynamics (shape, phase, membrane-pressure profile and curvature strain) and which are the implications for their biological activity? Epand had previously suggested that the ability of membrane active peptides to alter lipid polymorphism mainly resides on five factors: hydrophobicity, charge conformation and self-association, steric effects and mode of insertion [203,204]. Some examples of the formation of non-lamellar phases by antimicrobial peptides have been reported by some authors. Sevcsik et al. [205] have shown that LL-37 induces a peptide-associated quasi-interdigitated phase in negatively charged phosphatidylglycerol (PG) model membranes. Polyphemusin-1 and the variant PV5 were shown to decrease the lamellar to inverted hexagonal phase transition temperature of PE, indicating the induction of negative curvature strain and promotion of the HII phase [206]. The formation of this hexagonal phase could account for a lipid–peptide intermediate in the mechanism of action of these peptides. The authors have proposed that this peptide acts by a translocation mechanism involving five steps, specifically the initial electrostatic interaction with the anionic phospholipid membrane, the partition and insertion of the peptide into the hydrophobic core of the membrane, the peptide aggregation and formation of the non-bilayer intermediate with a random distribution of peptide between the core and the inner and outer leaflets of the membrane, collapse of this intermediate and redistribution of the peptide along both sides. The accumulation of the peptide in the cytoplasm, as observed by Powers et al. [132], would be explained on the basis of lipid composition asymmetry between the two leaflets of the membrane, which would account for the release of the peptide from the inner leaflet and accumulation in the cytoplasm [182]. Regarding the formation of cubic phases in particular, a wellknown antimicrobial peptide, gramicidin S (GS), has the ability to form such phases in relevant biological mimicking membrane systems [207,208]. Prenner et al. have initially studied the interactions of the cyclic peptide GS with single-component lipid bilayers and membrane polar lipid extracts of Acholeplasma (A.) laidlawii B and E. coli [208]. 31P nuclear magnetic resonance (NMR) spectroscopy and X-ray diffraction have revealed that the binding of GS to membranes solely composed of PC, PS, CL or SM presented an axially symmetric 31P NMR powder patterns throughout the entire temperature range studied, suggesting the absence or little effect on the membrane structure with respect to the formation of non-lamellar phases. However, in the presence of PE, PG or a nonlamellar phase-forming PC and even on the heterogeneous lipid mixtures of A. laidlawii B and E. coli membranes, an isotropic component is observed in the 31P NMR spectra at high temperatures, which is consistent with the formation of a cubic phase [209]. It was observed that the relative intensity of this component was increased with increasing temperature and GS concentration. Recently, Staudegger et al. have demonstrated that lipid extracts of these two bacteria induce the formation of inverted cubic phases of the space groups Pn3m and Im3m and that its formation was observed in physiologically-relevant conditions [210]. Therefore, the possibility of natural occurrence of ‘‘cubic phase domains’’ in the membrane and the rearrangement of the bilayer structure and dynamics by the peptide, leading to monolayer curvature stress and to formation of the cubic phase, could be assigned as a mechanism of action and concur to their biological activity. Zweytick et al. [83] have recently studied the interaction of human lactoferrin derivatives, VS1–13 and VS1–24, with E. coli total lipid extracts. The formation of non-lamellar phases was detected by SAXS (small-angle X-ray scattering), where two cubic phases of the space group Pn3m and Im3m could be assigned, coexisting with the lamellar phase. The ability to induce cubic phase formation was also correlated with the antimicrobial activity as the most potent antimicrobial peptide VS1–24 was a stronger promoter of cubic phases than its less active counterpart VS1–13. Therefore, it is tempting to assume that the formation of cubic phases and, more generally non-lamellar phases, may indeed be related to the mechanism of action of antimicrobial peptides and their potency towards pathogens. Interestingly, some authors have shown that some AMPs, such as protegrin-1, PGLa and gramicidin S, were also able to form cubic phases on pure POPE membranes, which is one of the main components of bacterial membranes. It was shown that GS had the strongest effect whereas melittin fully stabilized the lamellar phase [211]. Nisin was also shown to form cubic phases with POPE membranes and it was suggested that the insertion of the peptide in the bilayer shifts the amphiphilic balance by increasing the hydrophobic contribution, which would be the basis of the changes in the polymorphic state of PE. This was supported by the fact that the presence of cholesterol in the PE bilayer inhibits the membrane perturbation by nisin, most likely due to the presence of the sterol and its effect on membrane structure and fluidity [212]. Another study using X-ray diffraction and NMR has shown that the presence of 1% alamethicin introduces a large region of cubic phase into the phase diagram of bulk PE membranes [213]. It is highly conceivable that peptide-mediated non-lamellar phase formation in bacterial membranes may provide a mechanism of action and have biological consequences. Haney et al. have highlighted some important aspects on this concept [182]. The local destabilization of the membrane structure by altering lipid packing and sorting of different lipids seems plausible, particularly on PE-enriched domains with a natural propensity to form nonlamellar phases or regions with high negative curvature strain. In this context, Haney et al. propose that the different PE content on bacterial membranes and the extent of non-lamellar phase formation may be the basis of different bacterial susceptibilities to AMPs [182]. Epand et al. have recently reviewed the importance of membrane domains and the loss of their stability and functional properties upon binding of antimicrobial peptides. We suggest that this can be viewed in the light of non-lamellar phase induced formation, as it may induce an unstable membrane where such natural domains may lose their biological function due to the formation of a cubic phase and disorganization of ’functional’ lipid domains [137], in similar fashion as suggested for the peptide-induced lipid lateral and phase segregation mechanism. Moreover, bicontinuous cubic phases are characterized by water channels that weave their way through a single continuous bilayer equally divided into two inter-linked but separate aqueous sub-volumes. Therefore, the formation of this complex network of water channels across the structure may contribute to the leakage of ions and other metabolites and consequent loss of membrane function as a permeability barrier. Recently, a new concept has emerged that establishes a close relationship between alterations in the lipid structure and the modification of cell signaling events. Amphitropic enzymes comprise a class of proteins whose activities are modulated by the reversible translocation to membrane surfaces in response to local fluctuations on membrane dynamics and organization. This translocation may be regulated by the membrane lipid composition and by the membrane physical properties [199]. Two well-recognized examples of amphitropic enzymes that respond to lipid polymor- V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 phism are protein kinase C and CTP:phosphocholine cytidylyltransferase [199]. Protein kinase C (PKC) is a family of enzymes important in cell growth and proliferation, differentiation and cell signaling. PKC consists of several isoforms that require phospholipid, Ca2+ and diacylglycerol as cofactors for activation [214]. It is known that PKC activity is influenced by the presence of non-lamellar forming lipids and the basis of its binding to lipids has been recently reviewed [214]. Giorgione et al. have studied the activity and membrane binding of PKC in lipid bicontinuous cubic phases and hexagonal phases using MO/PS and DEPE/alamethicin as cubic phase lipid systems. These phases were shown to trigger the PKC-catalyzed phosphorylation of histone and it was further shown that the specific activity of the enzyme bound to cubic phase membranes is much greater than that bound to phospholipid in the lamellar phase. Since alamethicin is a well-known antimicrobial peptide produced by the fungus Trichoderma viride, the ability to alter the activity of an enzyme involved in cell signaling establishes a possible correlation between non-lamellar phase formation and modulation of cell signaling events. The capability to alter cell metabolism by other compounds, such as anticancer agents, have been recently reported in a representative study by Martínez et al. [215]. The anticancer drug, 2-hydroxy-9-cis-octadecenoic acid (Minerval) increases the tendency of PE membranes to organize into nonlamellar (hexagonal HII) phases, promoting the subsequent recruitment of protein kinase C (PKC) to the cell membrane with inhibition of the growth of cancer cells and antitumor effects in animal models of cancer without apparent histological toxicity [215]. Therefore, we can only speculate that the cubic phase triggered by antimicrobial peptides may possibly generate the very same effect, i.e. it can modulate important cell signaling pathways by altering the activity of amphitropic enzymes. This seems to be, at least, reasonable, although to the best of our knowledge, an example of an antimicrobial peptide that induces alterations on cell signaling in vivo has not yet been reported. From all the studies described, it is now clear that AMPs have the ability to modulate the physical properties of the membrane and, as a result of their interaction with the membrane, induce lipid phase and topological changes, alter the membrane curvature 167 strain and consequently the membrane morphology or modify the spatial arrangement of lipids and water molecules. All of these effects carry relevant in vivo implications on biological membrane structure and ultimately on cell viability. The functional and integral knowledge of such interactions is therefore of major importance in the elucidation of AMPs mechanisms of action upon binding to the membrane. The relationships that might be derived between the mechanism of action and its influence on membrane structure will lead to a more comprehensive understanding of the structure–activity paradigm for these peptides, which may be used in the design of novel compounds with improved properties. 6. Intracellular targets of antimicrobial peptides: an alternative mechanism of action? Another area of intensive focus regarding AMP biology has recently emerged, providing a new point of view to the mechanisms by which antimicrobial peptides cause cell death by acting by a non-permeabilizing mechanism or by targeting intracellular components [48]. For a long time, it was assumed that their antimicrobial action was mainly mediated by membrane disruption. In fact, AMPs were mainly assumed to act through the formation of transmembrane pores, leading to leakage of ions and metabolites, depolarization of the membrane potential and impairment of cellular energetics and viability. Currently, an increasing amount of studies has given evidence that membrane permeabilization alone appears insufficient to cause cell death by some AMPs and therefore other complementary or novel mechanisms have recently been reported (Table 2). Skleravaj et al. have demonstrated that several Bac5 and Bac7 fragments did not permeabilize E. coli although a decrease in the number of viable organisms could be observed [216]. One of the most interesting studies has shown the existence of AMP-mediated activation of proteases, such as phospholipase A2, whose activity is markedly enhanced by magainin 2, indolicidin and temporins B and L in the presence of calcium [217]. This synergistic activity has been pointed out by some authors as a major connection between AMPs and immune elements in an infection context [1]. Table 2 Intracellular components targeted by AMPs. The antimicrobial peptides buforin II and pleurocidin have been shown to inhibit DNA and RNA synthesis without disrupting the membrane. Protein synthesis is another macromolecular target for antibacterial peptides such as indolicidin and PR-39. Several antibacterial peptides have been shown to act on other intracellular processes, such as enzymatic activity. The ATPase activity of DnaK, an enzyme involved in chaperone-assisted protein folding, is targeted by pyrrhocidin, while inhibition of enzymes involved in the modification of aminoglycosides has also been demonstrated. Cell wall synthesis may also be compromised by the action of some peptides, such as mersacidin. Antimicrobial peptides DNA and cell division Buforin II [218], tachyplesin I [266], ABP-CM4 [267], polyphemusin [132],* pleurocidin and magainin 2 [268] Hexapeptide WRWYCR [221] dermaseptin [223], HNP -1 and -2 [269], PR-39 [270], indolicidin [271] and plant thionins [272] PR-39 [220], PR-26 [220], indolicidin [271] and microcin 25 [273] Enzymatic activity and protein synthesis Purothionin [274] Pyrrhocoricin [225], Bac7 [275] Pleurocidin [223], dermaseptin [223], HNP -1 and -2 [269], PR-39 [270], indolicidin [271] and plant thionins [272] * Intracellular target/mode of action DNA binding DNA repair enzymes Inhibition of nucleic-acid synthesis Septum formation Ribonucleotide reductase Inhibition of DnaK chaperone Inhibition of protein synthesis Cell wall Nisin Z [142], plectasin (fungal defensin) [276] and mesarcidin [277] plant antimicrobial protein-2 (Ac-AMP2), tachycitin and penaeidins [278] Cell wall precursor lipid II Chitin-binding activity Eukaryotic Organelles Histatin-5 [20] and hLF(1–11) [87] ABP-CM4 [267] and BMAP-28 [279] Cationic a-helical neuropeptides [280] Energetic metabolism impairment (mitochondria) Mitochondria Energetic metabolism failure (autophagic-like cell death) Possible direct PM1-biotin/DNA binding. 168 V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 For those peptides that gain access to intracellular targets, it is essential to cross the membrane and translocate to the cytoplasm. Buforin II, a linear AMP derived from proteolytic processing of histone H2A with a proline hinge, is an example of peptides that do not permeabilize membranes but still penetrate this physical barrier and accumulate in the cytoplasm [218]. The mechanism by which this peptide is translocated was revealed by combinatorial studies by fusing the proline-hinge region of buforin II with the N-terminal helix of magainin 2. It was shown that these hybrid peptides translocated bacterial cytoplasmic membranes and accumulated in the cytoplasm, yet presenting antimicrobial activity. Once in the cytoplasm, peptides usually concentrate and unleash many different processes, such as the alteration of septum formation in cytokinesis, inhibition of cell-wall, nucleic-acid and protein synthesis and suppress certain protein functions with an intrinsic metabolic cost [219]. PR-39 is a proline–arginine-rich neutrophil peptide and its Nterminal 1–26 fragment, PR-26, were shown to induce filamentation of Salmonella enterica serovar Typhimurium (Salmonella typhimurium). The deficiency in septum formation is characterized by an extremely elongated morphology and this may arise from the blocking of DNA replication or the inhibition of membrane proteins involved in septum formation [220]. More recently, Su et al. have demonstrated that a hexapeptide, WRWYCR was capable of inhibiting S. typhimurium growth within murine macrophages [221]. This peptide acts by inhibiting unrelated DNA repair enzymes, thus trapping and preventing resolution of the Holliday junction intermediate. In general, WRWYCR and its D-stereoisomer, wrwycr, were shown to be bactericidal against both Gram-positive and Gram-negative bacteria by promoting DNA breaks, chromosome segregation defects and filamentation of cells [221]. The antimicrobial peptide, microcin B17, is also believed to inhibit an intracellular target within E. coli. This peptide has been suggested to specifically inhibit DNA replication by targeting DNA gyrase [222]. At the MIC, pleurocidin and dermaseptin both inhibit nucleic acid and protein synthesis without damaging the E. coli cytoplasmic membrane [223]. A study by Gifford et al. has demonstrated that Lfcin B localizes in the cytoplasm and inhibits macromolecular synthesis of DNA, RNA, and proteins [224]. Kragol et al. [225] have recently showed that the insect antibacterial peptides pyrrhocoricin, drosocin and apidaecin bind to the bacterial heat shock protein DnaK, and this inhibition is associated with cell death since binding DnaK prevents chaperone-assisted protein folding and inhibits its associated ATPase activity by impairing the peptide-binding pocket of DnaK. The peptide permanently closes the cavity and inhibits chaperone-assisted protein folding. An important feature that is usually disregarded is the action of antimicrobial peptides on eukaryotic organelles, which has proven to be important in immunological resolution of fungal infections and even in cancer cases. One such example is the activity displayed by some lactoferrin derived-peptides. For instance, human lactoferricin hLF (1–11) binds to a receptor on the fungal cell membrane, accumulates in the cytoplasm and induces the loss of ATP and the dissipation of the electrochemical gradient by targeting energized mitochondria [87,226]. Helmerhorst et al. found that the cationic peptide histatin-5 binds via Ssa1/2p surface protein, enters the cell and causes a depletion in mitochondrial membrane potential in Candida albicans [20]. Another example was found by Mader et al. who described that LfcinB is able to permeabilize the membrane without immediate disruption and mainly targets mitochondria, inducing apoptosis in Jurkat T-leukemia cells by loss of transmembrane potential and cytochrome c release [227]. Although the number of examples of AMPs intracellular action is growing in the literature, the information available is still scarce and does not yet allow a comprehensive and a clear perspective on the mode of action of AMPs on these molecular and cellular targets. 7. Mechanisms of antimicrobial peptide resistance In order to survive in a specific environment, pathogens have developed several mechanisms of resistance to counteract the host defense. This is important in the context of the present review with regards to the role of antimicrobial peptides in innate immunity. The current view states that such mechanisms are difficult to develop since the action of the majority of antimicrobial peptides is quite unspecific, targeting mostly the negatively-charged membrane of pathogens, when compared with that of most classic antibiotics, which have more specific molecular targets [14]. In the case of AMPs, the resistance would involve an overall organization of the whole membrane beyond the capacity for the pathogen to rearrange the cell wall and maintain its viability. However, a variety of studies have already documented the existence of some resistance mechanisms to a given peptide through constitutive or inducible mechanisms in a diverse and dynamic manner. These bacterial strategies target some key steps, such as the antimicrobial peptide attachment, peptide insertion and membrane permeability. 7.1. Membrane electrostatics and structural modifications Part of the unspecific action of antimicrobial peptides arises from their electrostatic interaction with the anionic phospholipids of the membrane, which simultaneously confers its selectivity towards pathogens [24]. Therefore, one of the evolutionary mechanisms found by microorganisms consists on a membrane with a reduced net negative charge without leading to significant modification of membrane fluidity or enzymatic activities (Fig. 9) [228]. Early studies by Dorrer and Teuber have shown that some pathogens such as Pseudomonas fluorescens adaptively change the electronegativity of the cytoplasmic membrane by decreasing the content of anionic phospholipids (PG and CL) and increasing cationic ornithine–amide lipid composition when incubated in a poor phosphate limited medium and that this induces a resistance to cationic polymyxin B [229]. On the other hand, some bacteria modified the membrane by increasing the content of cationic constituents as observed on some S. aureus strains. They present an unusual resistance to defensins and protegrins and such feature has been linked to lysine modification of PG in the cytoplasmic membrane, forming lysyl-PG, which appears to be under the mprF operon control [230]. The teichoic acid polymers found in the cell walls of Gram-positive bacteria have strong anionic properties as all the phosphate groups in their glycerolphosphate repeating units contain negative charges. Streptococcus agalactiae and Listeria monocytogenes partially neutralize this negative charge by modifying teichoic acid with D-alanine residues that bear positively charged amino groups by the products of the dltABCD operon [231,232]. The presence of a capsular polysaccharide or glycocalyx shielding the pathogens from AMPs and other compounds is undoubtedly one of the most effective means of circumventing hostdeployed agents (Fig. 9) [24]. Capsular polysaccharides, formed from the oligomerization of anionic monomer subunits, are typically negatively charged and therefore can bind antimicrobial peptides and partially shield the cytoplasmic membrane from peptide action. P. aeruginosa exhibits an unusual propensity to infect tissues in which dysfunctional salt transport results in abnormal tissue physiology, abnormal phagocyte function, and increased local ionic strength, as it can be seen in some pathologies like cystic fibrosis [27]. A study by Friedrich et al. [233] suggests that the glycocalyx of alginic acid, an anionic and the main capsular exopolysaccharide constituent produced by virulent strains of P. aeruginosa, is necessary to sequester cationic antimicrobial pep- V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 169 Fig. 9. Antimicrobial peptide mechanisms of resistance. The most common and specific mechanisms of resistance triggered by pathogens to decrease their susceptibility to AMPs are described. tides present in mucosal secretions, before they can achieve threshold concentrations and reach the membrane, thereby conferring partial resistance to the membranolytic action of the peptides. AMPs initially target and interact with microbial structures exterior to the cytoplasmic membrane. Thus, microbial pathogens have developed mechanisms by which these targets may be modified to resist peptide targeting and circumvent the resulting antimicrobial mechanisms. One of the most important mechanisms is the particular susceptibility of Gram-negative bacteria to peptides that specifically target LPS (Fig. 9). The alteration of the structure of LPS through mechanisms such as lipid A acylation, 4-amino-4deoxy-L-arabinose and palmitate derivation of lipid A, aminoarabinose and myristoylation in E. coli have been reported as mechanisms of resistance employed by some Gram-negative bacteria. For instance, resistance to polymyxins has been consistently linked to the presence of LPS with a less anionic lipid A, to acylation with an additional fatty acid, or to the presence of an O antigen [234]. Phosphorylcholine is a component of LPS in some bacteria and may mimic PC present in mammalian cell membranes, which are usually less susceptible to AMPs activity. Haemophilus influenzae was shown to exhibit resistance to cationic antimicrobial peptide exposure correlated with the relative amount of phosphorylcholine present on its surface. Interestingly, this species resisted killing by antimicrobial peptides only when choline was provided, which is necessary for phosphorylcholine modification of LPS. Such feature was evidenced by Lysenko et al. that showed that H. influenzae ex- presses unusually high levels of phosphorylcholine and exhibited decreased susceptibility to the antimicrobial peptide LL-37/ hCAP18 expressed in the upper respiratory tract [235]. Such envelope modifications are important to understand their impact as a mechanism of resistance and represent a reliable strategy in microbial resistance to antimicrobial peptide-based immune mechanisms in medical pathology. This interesting aspect provides further evidence of the specificity of the action of AMPs and the ability of microorganisms to take evolutionary advantage of such discrimination to avoid or, at least, decrease their susceptibility to AMPs. In addition, these findings emphasize the difficulty to access biological relevant processes in vitro, since resistance in vivo is dependent on strategies and specific microenvironments relevant to immunoavoidance. 7.2. Membrane electrical potential The main mechanism by which cationic antimicrobial peptides may selectively target pathogen cells is the electrostatic adsorption due to their higher negative electrochemical potential [4,24]. Indeed, the presence of a transmembrane electric potential can lower the free energy of the insertion state relative to the surface adsorption state for peptides possessing a dipole moment, such as the case of helical peptides. Although this might seem to be a biophysical parameter that would necessarily remain constant, a number 170 V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 of studies now suggest that some organisms may evade host-defense mechanisms by modulating this parameter. Early studies with the type-I (highly cationic) and type II adefensins have demonstrated that the antimicrobial activity occurs in a different way for the two peptides since the last class exhibits maximum activity against highly energized cells [236]. Therefore, the magnitude of the electronegative potential of the membrane is important for the action of some antimicrobial peptides and it may explain the different pattern of action of related peptides or why some peptides are inactive against some strains. Some S. aureus strains with constitutive reductions in DW display reduced susceptibilities to some but not all platelet microbicidal peptides tested, as reported by Yeaman et al. [237]. However, there are some peptides that are insensitive to the transmembrane potential, as observed for protegrin-like peptides, which present no dipole moment [4238]. This aspect is particularly important because this ability to subvert the membrane potential and compromise the bacterial integrity and survival can be of major importance for the design and rational use on therapeutics against a specific set of microorganisms. Despite the fact that the cell metabolic status affects the transmembrane potential of the membrane, there are also other molecular targets (e.g. organelles) whose membrane energetics accounts for the mechanism of action of some peptides. This antimicrobial peptide resistance pattern has been observed in the eukaryotic cells like C. albicans. Gyurko et al. [239] have shown that mutants of C. albicans deficient in respiration resulting from mutations in mitochondrial DNA were drastically more resistant to histatin-5 when compared with their parental counterpart and that the bactericidal activity was significantly lower against the parental strain in the presence of inhibitors of respiration. These findings suggest that the anticandidal activities of histatin-5 require a threshold level of cellular energetics involving mitochondrial ATP synthesis, that is, energized mitochondria are required to exert its action. hLF(1–11) was reported to target energized mitochondria in C. albicans by interacting with the inner mitochondrial membrane, thus affecting mitochondrial output, e.g., generation of ATP and reactive oxygen species (ROS) [87]. 7.3. Sensor-transducer response systems One of the most ancient mechanisms of resistance used by microorganisms to face environmental challenges such as variations on ionic strength, pressure and temperature, is the existence of very sophisticated sensor-transducer response systems through which they alter their genetic and metabolic pattern until favorable conditions are reinstated. The finding that this kind of systems also exists as a mechanism to resist antimicrobial peptide stress was reported by Hong et al. [240], who demonstrated that sublethal concentrations of cecropin A prompted a pattern of genomic response that is distinguishable from that of lethal concentrations and distinct from global stress response systems such as heatshock or oxidative response, stressing the importance of genomic induction in sensing and prompting a coordinate response to AMPs’ exposure (Fig. 9). Studies of PhoP/PhoQ regulon in Salmonella and most recently the finding of YdeI/OmdA and its interaction with OmpD/NmpC in the same genera are representative of this type of mechanisms. Indeed, it was shown that Salmonella enterica presents at least three different sensor-kinase systems to modify gene expression in the presence of AMPs, namely PhoP-PhoQ, PmrA-PmrB, and RcsB-RcsC-RcsD. In the first case, the regulon comprises a sensor kinase, PhoQ, and a transcriptional activator, PhoP, which is encoded in response to environmental signals including changes in extracellular magnesium or calcium concentrations, pH or even after infection (phagocytosis). Besides, it encodes inducible surface and secretor enzymes that modify lipopolysaccharide, lipid and protein constituents of the outer membrane as well as proteases that likely degrade certain AMPs. PhoP–PhoQ also regulates the PmrA–PmrB two-component regulatory system, which is involved in the resistance of P. aeruginosa to polymyxin B and cationic antimicrobial peptides from human neutrophils in low-Mg2+ conditions. This is due to amino acid substitutions in the regulatory protein PmrA, which confers resistance to polymyxin by decreasing the overall negative charge of the lipopolysaccharide (LPS), which in turn lowers the affinity for AMPs and other cationic peptide antibiotics [241]. The ydeI gene is regulated by the RcsB-RcsC-RcsD pathway and encodes a 14-kDa predicted oligosaccharide/oligonucleotide binding-fold (OB-fold) protein important for polymyxin B resistance in virulence in mice. ydeI is additionally regulated by the PhoP-PhoQ and PmrA-PmrB sensor-kinase systems, which confer resistance to cationic AMPs by modifying lipopolysaccharide (LPS). Two independent biochemical methods found that YdeI co-purifies with OmpD/NmpC, a member of the trimeric b-barrel outer membrane general porin family. Genetic analysis indicates that OmpD contributes to polymyxin B resistance, and both YdeI and OmpD are important for resistance to cathelicidin antimicrobial peptide. YdeI is localized in the periplasm, where it could interact with OmpD. A second predicted periplasmic OB-fold protein, YgiW, and OmpF, another general porin, also contribute to polymyxin B resistance [242]. 7.4. Proteases and peptidases A growing number of microorganisms have presented a large set of proteolytic agents that cleave host-derived AMPs (Fig. 9), which is pointed out to become one of the most important mechanisms of resistance to AMPs and a great challenge in the future when these peptides will be translated into therapeutics. As described above, PhoP/PhoQ-like systems regulate many metabolic strategies employed by bacteria to resist antimicrobial peptides and trigger coordinate responses in order to counteract the peptide action. Among these, the PgtE protein was recently demonstrated by Guina et al. to be an outer membrane endopeptidase in Salmonella that confers resistance to a-helical AMPs [243]. Thus, a variety of amphipathic and cationic antimicrobial peptides are potential substrates of PgtE protease. However, PgtE protease does not confer Salmonella increased resistance to antimicrobial peptides exhibiting amphipathic b-sheet conformation induced by intramolecular disulfide bonds (e.g. defensins or protegrins). This probably creates a steric hindrance that is protective against the activity of PgtE protease. More recently, this protease was shown to affect the complement system activity, which further extends its activity on other immune branches in order to subvert the host defense system [244]. Other proteases have also been implicated in AMPs resistance of S. aureus and E. coli [245], namely the heatshock serine protease DegP that seems to confer a considerable resistance to E. coli in the presence of lactoferricin B in vitro. Due to limitations on peptide proteolytic stability, a number of structural modifications have been proposed to overcome this problem, leading to enhanced AMP biological lifetimes and therapeutic index. For this purpose, natural and even synthetic AMPs with specific structures were submitted to structural modifications conferring resistance to proteolytic degradation. One of the most prominent examples derives from indolicidin, a small cationic peptide amide composed of 13 amino acids, five of which are tryptophan residues. The group of Ösapay et al. has built a synthetic modified form of indolicidin, named X-indolicidin, which was produced by deprotonation of two indole side chains, yielding an intrachain ditryptophan configuration in which the Trp-6 and Trp-9 residues are covalently linked. This variant X-indolicidin V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 was shown to be more resistant to trypsin and chymotrypsin digestion, suggesting that ditryptophan stabilizes an indolicidin conformer resistant to certain proteases [246]. More recently, Fernández-Reyes et al. have produced various modified lysinetrimethylated analogues of a cecropin A-mellitin hybrid [CA(1– 7)M(2–9)], which exhibits an improved proteolytic (trypsin cleavage) stability profile, although protease shielding does not seem to be the only contributing factor to the improved performance of the constructed trimethylated analogues [184]. Overall, these studies have provided evidence that natural and engineering-improved synthetic AMPs can be a very useful tool to increase peptide stability in solution for future therapeutical purposes. 7.5. Efflux-dependent resistance mechanisms Porin-mediated efflux is widely studied as a relevant resistance mechanism to classical antibiotics in order to avoid intracellular accumulation and the possibility to reach a threshold concentration after which they exert their antibacterial action. Recently, it has also emerged as a mechanism by which microbial pathogens may oppose antimicrobial peptide action (Fig. 9). Early studies by Shafer et al. provided evidence of the existence of this kind of mechanism. In Neisseria gonorrhoeae, the resistance to AMPs is mediated in part by an energy-dependent efflux system termed mtr [247]. Evidence indicates the MtrCDE complex mediates the efflux of antibiotics, dyes and detergents, suggesting that this mechanism also protects the pathogen against AMPs within and beyond the genitourinary tract. A similar mechanism was also observed in Yersinia and the efflux of AMPs appears to be mediated by a temperature-regulated system involving the efflux pump/potassium antiporter complex formed by the RosA and RosB proteins [248]. Importantly, the RosA/RosB gene regulon appears to be specific, since it is inducible in the presence of AMPs and in vivo, and it has been suggested to enhance the survival of the organism in the acidic and antimicrobial peptide-rich phagolysosome in polymorphonuclear leukocytes. The presence of efflux systems was also found in Grampositive bacteria and fungal pathogens. For example, the plasmid encoded gene qacA mediates staphylococcal-resistance to multiple organic cations, like AMPs and polyamines, via a proton motive force-dependent efflux pump. A study by Kupferwasser et al. demonstrated that an S. aureus plasmid containing qacA provides resistance to antimicrobial peptide tPMP-1 [249]. Notably, the expression of qacA did not appear to impart cross-resistance to other structurally distinct cationic peptides, including a defensin, protamine, or the lantibiotics pep5 or nisin. More recently, Bina et al. have provided evidence that RND efflux systems in Vibrio cholerae were responsible for resistance to AMPs [250]. The existence of such efflux pumps illustrates the importance of these systems, not only to block the passage of classical antibiotics but also to provide a means of protection by which antimicrobial peptides are not allowed to reach lethal concentrations and to trigger their biological effects. 8. Development of antimicrobial peptides for clinical applications: a novel advance in therapeutics There is a very alarming increase in pathogenic microorganisms that are multi-resistant to commercially available antibiotics, some of them acquiring resistance in very short periods of time (decades) [6,8,10,14,49,135,140,228]. Vancomycin resistant enterococci (VRE) and methicillin resistant S. aureus (MRSA) are occurring with increasing frequency and represent a serious threat in hospitals worldwide. In the last 25 years, MRSA incidence has raised more than 10-fold and VRE has witnessed similar increases 171 within the last 15 years [73]. This has been attributed to the excessive (and often inappropriate) use of antibiotics in human and animal health care for the treatment and prevention of infections, as well as, to the increased use of immunosuppressive chemotherapeutic regimes. Indeed, the last statistics of the World Health Organization have highlighted a very important and serious global health problem that has pressed the scientific community to develop novel antibiotics with improved properties and mechanisms of action. Nowadays, the situation presents itself very disquieting because only few antibiotics that provide successful response to infection are available; during the period of 1983–1987 only 16 new compounds appeared, and that number decreased to only 7 between 1998 and 2002 [166]. For some of them, studies have already documented mechanisms of resistance in a few microorganisms [166]. To face this situation, considerable progress has been made in the field of microbial genomics which has provided a more comprehensive understanding of the mechanisms to countermeasure the action of host immune response. The development of molecular biology tools brought forth a number of strategies for creating antibiotics with better therapeutic index, such as metalloantibiotics, the new generation of macrolide antibiotics, or even the development of adhesin-based vaccines, which induce antibody responses at the mucosal surface that prevent attachment and abrogate colonization, and therefore block the primary stages of infection [251]. Another emerging strategy is based on host antimicrobial peptides that can provide a first line of defense against a wide range of pathogenic microorganisms. For the therapeutics purpose, a new array of natural and synthetic antibiotics derived from combinatorial libraries has emerged as a reliable alternative to overcome the ultimate problem of worldwide antibiotic resistance. The potential therapeutic applications for AMPs or derived products may arise from the amplification of antimicrobial therapy when conjugated with themselves or with conventional antibiotics [25,28,252]. Such synergism has already been applied for classic antibiotics in order to improve the effectiveness of the treatment, particularly for multi-resistant strains. For instance, the use of sulfonamides and diaminopiridines (trimethoprim) allow the inhibition of tetrahydrofolic acid necessary for nucleic acids and protein synthesis. An obvious application of these peptides comes into view given their propensity to permeabilize target microbial membranes and form transient or permanent pores, which may facilitate conventional agents in overcoming surface resistance mechanisms such as reduced uptake or enhanced efflux through microbial pumps. On the other hand, some synergistic interactions between peptides themselves have proved to be efficient in overcoming bacterial resistance, e.g. in that imposed by the LPS leaflet of Gram-negative bacteria, like the combined use of closely related temporins A/ B and L [25]. A study by Giacometti et al. has demonstrated the potential of combinational therapy, such as the verified synergy of cecropin A-melittin hybrid peptides with b-lactams [253]. Moreover, some peptides act only at the intracellular level and may also be used in conjugation with classical antibiotics, like buforin II (DNA) and fluoroquinolones (DNA gyrase or topoisomerase IV), amplifying its antimicrobial action on a specific or similar target. Current knowledge regarding the relationship between peptide structure and function as well as the mechanism of action has been applied in the rational design of antimicrobial peptide variants with a broad spectrum of activity. However, in spite of its large potential as therapeutic agents in many areas [6,7,10,13,26,254], the development of such agents has proved to be difficult due to important aspects concerning its manufacture, pharmacokinetics, site of action delivery and specific properties concerning the class of microorganisms they are active against. Furthermore, their stability in the infection context still remains an issue, since most 172 V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 studies reported are mainly performed in vitro without taking into account the very specific physiological environment of infection in which antimicrobial peptides act on. 8.1. A promising future for antimicrobial peptides as reliable antibiotics? Presently, several studies have pointed out the exceptional potential of antimicrobial peptides as universal and encouraging antibiotics for the treatment of many diseases, such as inflammatory and auto-immune diseases. However, their applicability in the market has proceeded at a slow rate and less than a few hundred peptides have been evaluated to date for clinical potential, a number that is quite small compared to the figures for many antibiotic development programs, particularly classical antibiotics. Despite the promising attributes and recent successes in demonstrating the efficacy of antimicrobial peptides already in animal disease models, there are considerable challenges in the clinical application of candidate peptide therapies. Some of these obstacles were already summarized, namely the cost of peptide synthesis since it is an expensive and time-consuming process [44]. Besides, some difficulties have already been mentioned, e.g. the susceptibility of peptides to proteolytic degradation in vivo and its consequences on the pharmacokinetics and interaction with pathogen, the lack of consistent information regarding the potential toxicity of amphipathic peptides to host cells, etc. [44]. Nevertheless, probably the most important feature relies on lack of consistent knowledge on adequate antimicrobial activity of natural and synthetic AMPs under specific physiological conditions. There has been limited success with those antimicrobial peptides that have entered into clinical trials because no peptide has yet been approved by Food and Drug Administration (FDA) for clinical applications. The most advanced AMPs are currently two indolicidin-based antimicrobial peptides, MBI-226 and MX-594AN that have been developed by Migenix (Vancouver, British Columbia, Canada) for the treatment of catheter-related infections and acne, respectively. MBI-226 (Omiganan) showed promising results for secondary end points in phase IIIa trials, resulting in a 40% reduction of catheter colonization and a 50% decrease in tunnel infections, although its primary end point of a reduced rate of infections was not achieved. Another known peptide with clinical trials currently underway is IB-367 (Iseganan), a protegrin-derived peptide targeted for oral mucositis in cancer patients (Intrabiotics Pharmaceuticals, Inc. Mountain View, CA). Based on the pig peptide protegrins, IB-367 possesses broad-spectrum activity in vitro against bacteria and fungi; however, in clinical trials it failed to prevent or reduce oral mucositis compared with a placebo. Unfortunately, increased rates of pneumonia and mortality were observed in patients receiving the peptide, which eventually halted the clinical trials. AM-Pharma Holding BV have announced that they have completed phase I clinical trials with the 11-mer peptide from the Nterminus of human lactoferrin, hLF (1–11). It was shown that this peptide was effective in animal models of osteomyelitis and other bacterial infections. AM-Pharma is also targeting the prevention of infections in patients undergoing stem cell, especially allogeneic transplantation, with common high post-operative rates of severe infection and mortality. The treatment of patients with hematological malignancies by means of hematopoietic stem cell transplantation (HSCT) has been recently considered [255]. Similarly, DermaGen AB (Pergamum) has received promising results from a clinical Phase I/IIa study of a novel antimicrobial peptide (DPK060) treatment for atopic dermatitis. The peptide has shown a broad spectrum of activity and exhibited both bactericidal and fungicidal activities. In the clinical trial, the company’s candidate drug clearly reached its primary objective, demonstrating a significant reduction of total microbes in eczemas compared to placebo. In addition to good safety and tolerability performance, the candidate drug also showed a trend towards improved eczema status (the research was performed in the website http://www.dermagen.se/ at 20th February 2011). The 21 kDa recombinant N-terminal fragment of Bactericidal/ permeability-increasing protein (BPI), rBPI21, has recently completed Phase III clinical trials of parenteral use for meningococcaemia and it was shown to be also a promising agent against septic shock [256]. Although none of the peptides in the examples described above have obtained the approval from FDA for clinical use, the accumulation of evidence so far has enhanced the optimism on the conceptualization of antimicrobial peptides as encouraging therapeutic agents in the prevention and management of diverse clinical conditions. 9. Concluding remarks and future directions The study of antimicrobial cationic host defense peptides in the past few years has shown their importance as therapeutic weapons. Many studies have provided consistent evidence that these peptides have such potential as they display activity towards a wide spectrum of infectious bacterial, viral, fungal, and parasitic pathogens and even cancer cells. In addition, many reports have claimed diverse immunomodulatory activities of such host defense peptides, providing an additional incentive when considering these peptides as a new class of therapeutic agents. The structure–activity studies have identified common traits among their properties, namely their cationic nature and amphipathic structure, which in part justify their action and selectivity towards pathogens. Differences in biochemical and biophysical properties of pathogens versus host cells provide an additional basis for their selective toxicity. Moreover, many mechanisms of action have provided insight into how the peptides target the membrane either by promoting its permeabilization, altering its topology or by crossing it in order to gain access to intracellular targets, such as nucleic acids, enzymes or even eukaryotic organelles. However, some microorganisms were successful in circumventing this host defense mechanism and the understanding of the balance between the action of the peptide and the resistance mechanisms triggered by the pathogen will provide further evidence for the evolutionary maintenance of this ancient branch of immunity. These insights may provide novel strategies or templates from which new peptides can be designed to improve the prevention or treatment of infections. Overall, the comprehensive knowledge of the elusive aspects of their mechanism of action and the improvement of their properties will definitely contribute to consolidate their potential and the claims of these peptides as the next-generation antibiotics to prevail over the alarming worldwide antibiotic crisis. Acknowledgments The authors acknowledge Fundação para a Ciência e Tecnologia (FCT-Portugal) for financial support to CIQ(UP), Unidade de Investigacão 81 and to REQUIMTE (Rede de Química e Tecnologia). Dr. S. Connell, University of Leeds, UK, is gratefully acknowledged for assistance during the reviewing process of this manuscript. References [1] Bowdish DME, Davidson DJ, Hancock REWH. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr Protein Pept Sci 2005;6:35–51. [2] Diamond G, Beckloff N, Weinberg A, Kisich KO. The roles of antimicrobial peptides in innate host defense. Curr Pharm Des 2009;15:2377–92. V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 [3] Hancock R, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol 2000;8:402–10. [4] Huang HW. Action of antimicrobial peptides: two-state model. Biochemistry 2000;39:8347–52. [5] Stella L, Mazzuca C, Venanzi M, Palleschi A, Didonè M, Formaggio F, et al. Aggregation and water-membrane partition as major determinants of the activity of the antibiotic peptide trichogin GA IV. Biophys J 2004;86:936–45. [6] Lata S, Sharma B, Raghava G. Analysis and prediction of antibacterial peptides. BMC Bioinformatics 2007;8:263. [7] Jeong K-W, Shin S, Kim J-K, Kim Y. Antibacterial activity and synergism of the hybrid antimicrobial peptide, CAMA-syn. Bull Korean Chem Soc 2009;30:1839–44. [8] Shin SY, Yang S-T, Park EJ, Eom SH, Song WK, Kim JI, et al. Antibacterial, antitumor and hemolytic activities of alpha-helical antibiotic peptide, P18 and its analogs. J Pept Res 2001;58:504–14. [9] Wachinger M, Kleinschmidt A, Winder D, von Pechmann N, Ludvigsen A, Neumann M, et al. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J Gen Virol 1998;79:731–40. [10] Glukhov E, Stark M, Burrows LL, Deber CM. Basis for selectivity of cationic antimicrobial peptides for bacterial versus mammalian membranes. J Biol Chem 2005;280:33960–7. [11] Papo N, Shai Y. Can we predict biological activity of antimicrobial peptides from their interactions with model phospholipid membranes? Peptides 2003;24:1693–703. [12] Sood R, Kinnunen PKJ. Cholesterol, lanosterol, and ergosterol attenuate the membrane association of LL-37(W27F) and temporin L. Biochim Biophys Acta (BBA) – Biomembr 2008;1778:1460–6. [13] Zhao H, Mattila J-P, Holopainen JM, Kinnunen PKJ. Comparison of the membrane association of two antimicrobial peptides, magainin 2 and indolicidin. Biophys J 2001;81:2979–91. [14] Jiang Z, Vasil AI, Hale JD, Hancock REW, Vasil ML, Hodges RS. Effects of net charge and the number of positively charged residues on the biological activity of amphipathic [alpha]-helical cationic antimicrobial peptides. Pept Sci 2008;90:369–83. [15] Hancock REW, Chapple DS. Peptide antibiotics. Antimicrob Agents Chemother 1999;43:1317–23. [16] Brown KL, Hancock REW. Cationic host defense (antimicrobial) peptides. Curr Opin Immunol 2006;18:24–30. [17] Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers 2002;66:236–48. [18] Hoskin DW, Ramamoorthy A. Studies on anticancer activities of antimicrobial peptides. Biochim Biophys Acta (BBA) – Biomembr 2008;1778:357–75. [19] Zasloff M. Antimicrobial peptides of multicellular organisms. Nature 2002;415:389–95. [20] Helmerhorst EJ, Breeuwer P, Van ‘t Hof W, Walgreen-Weterings E, Oomen LCJM, Veerman ECI, et al. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J Biol Chem 1999;274:7286–91. [21] Mochon AB, Liu H. The antimicrobial peptide histatin-5 causes a spatially restricted disruption on the Candida albicans surface, allowing rapid entry of the peptide into the cytoplasm. PLoS Pathog 2008;4:1–12. [22] Oudhoff MJ, Bolscher JGM, Nazmi K, Kalay H, Van ‘t Hof W, Amerongen AVN, et al. Histatins are the major wound-closure stimulating factors in human saliva as identified in a cell culture assay. FASEB J 2008;22:3805–12. [23] Broekaert WF, Terras F, Cammue B, Osborn RW. Plant defensins: novel antimicrobial peptides as components of the host defense system. Plant Physiol 1995;108:1353–8. [24] Yount NY, Bayer AS, Xiong YQ, Yeaman MR. Advances in antimicrobial peptide immunobiology. Pept Sci 2006;84:435–58. [25] Rosenfeld Y, Barra D, Simmaco M, Shai Y, Mangoni ML. A synergism between temporins toward gram-negative bacteria overcomes resistance imposed by the lipopolysaccharide protective layer. J Biol Chem 2006;281:28565–74. [26] Melo MN, Ferre R, Castanho MARB. Antimicrobial peptides: linking partition, activity and high membrane-bound concentrations. Nat Rev Microbiol 2009;7:245–50. [27] Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 2003;55:27–55. [28] Scott MG, Yan H, Hancock REW. Biological properties of structurally related [alpha]-helical cationic antimicrobial peptides. Infect Immun 1999;67:2005–9. [29] Mookherjee N, Hancock R. Cationic host defence peptides: Innate immune regulatory peptides as a novel approach for treating infections. Cell Mol Life Sci 2007;64:922–33. [30] Kruse T, Kristensen H-H. Using antimicrobial host defense peptides as antiinfective and immunomodulatory agents. Expert Rev Anti Infect Ther 2008;6:887–95. [31] Suzuki K, Murakami T, Kuwahara-Arai K, Tamura H, Hiramatsu K, Nagaoka I. Human anti-microbial cathelicidin peptide LL-37 suppresses the LPS-induced apoptosis of endothelial cells. Int Immunol 2011;23:185–93. [32] Ghiselli R, Cirioni O, Giacometti A, Mocchegiani F, Orlando F, Bergnach C, et al. Effects of the antimicrobial peptide BMAP-27 in a mouse model of obstructive jaundice stimulated by lipopolysaccharide. Peptides 2006;27: 2592–9. [33] Giacometti A, Cirioni O, Ghiselli R, Mocchegiani F, Del Prete MS, Viticchi C, et al. Potential therapeutic role of cationic peptides in three experimental models of septic shock. Antimicrob Agents Chemother 2002;46:2132–6. 173 [34] Gough M, Hancock R, Kelly N. Antiendotoxin activity of cationic peptide antimicrobial agents. Infect Immun 1996;64:4922–7. [35] Hirata M, Zhong J, Wright SC, Larrick JW. Structure and functions of endotoxin-binding peptides derived from CAP18. Prog Clin Biol Res 1995;392:317–26. [36] Gudmundsson GH, Agerberth B. Neutrophil antibacterial peptides, multifunctional effector molecules in the mammalian immune system. J Immunol Methods 1999;232:45–54. [37] Lillard JW, Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc Natl Acad Sci USA 1999;96:651–6. [38] Brogden KA, Heidari M, Sacco RE, Palmquist D, Guthmiller JM, Johnson GK, et al. Defensin-induced adaptive immunity in mice and its potential in preventing periodontal disease. Oral Microbiol Immunol 2003;18:95–9. [39] Yang D, Biragyn A, Kwak LW, Oppenheim JJ. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol 2002;23:291–6. [40] Easton DM, Nijnik A, Mayer ML, Hancock REW. Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol 2009;27:582–90. [41] Tossi A, Sandri L, Giangaspero A. Amphipathic, [alpha]-helical antimicrobial peptides. Biopolymers 2000;55:4–30. [42] Giangaspero A, Sandri L, Tossi A. Amphipathic [alpha]-helical antimicrobial peptides. Eur J Biochem 2001;268:5589–600. [43] Jenssen H, Hamill P, Hancock REW. Peptide antimicrobial agents. Clin Microbiol Rev 2006;19:491–511. [44] Marr AK, Gooderham WJ, Hancock REW. Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol 2006;6:468–72. [45] Huang Y, Huang J, Chen Y. Alpha-helical cationic antimicrobial peptides: relationships of structure and function. Protein Cell 2010;1:143–52. [46] Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta (BBA) – Biomembr 1999;1462:11–28. [47] Wiesner J, Vilcinskas A. antimicrobial peptides: the ancient arm of the human immune system. Virulence 2010;1:440–64. [48] Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 2005;3:238–50. [49] Watson JL, Gillies ER. Amphipathic b-Strand mimics as potential membrane disruptive antibiotics. J Org Chem 2009;74:5953–60. [50] Matsuzaki K. Why and how are peptide-lipid interactions utilized for selfdefense? Magainins and tachyplesins as archetypes. Biochim Biophys Acta (BBA) – Biomembr 1999;1462:1–10. [51] Tomasinsig L, Benincasa M, Scocchi M, Skerlavaj B, Tossi A, Zanetti M, et al. Role of cathelicidin peptides in bovine host defense and healing. Probiot Antimicrob Proteins 2010;2:12–20. [52] Lequin O, Bruston F, Convert O, Chassaing G, Nicolas P. Helical structure of dermaseptin B2 in a membrane-mimetic environment. Biochemistry 2003;42:10311–23. [53] Pukala TL, Brinkworth CS, Carver JA, Bowie JH. Investigating the importance of the flexible hinge in caerin 1.1: solution structures and activity of two synthetically modified caerin peptides. Biochemistry 2004;43:937–44. [54] Lee JY, Boman A, Sun CX, Andersson M, Jornvall H, Mutt V, et al. Antibacterial peptides from pig intestine: isolation of a mammalian cecropin. Proc Natl Acad Sci USA 1989;86:9159–62. [55] Holak TA, Engstroem A, Kraulis PJ, Lindeberg G, Bennich H, Jones TA, et al. The solution conformation of the antibacterial peptide cecropin A: a nuclear magnetic resonance and dynamical simulated annealing study. Biochemistry 1988;27:7620–9. [56] Silvestro L, Axelsen PH. Membrane-induced folding of cecropin A. Biophys J 2000;79:1465–77. [57] Bechinger B. Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. J Membr Biol 1997;156:197–211. [58] Matsuzaki K, Harada M, Handa T, Funakoshi S, Fujii N, Yajima H, et al. Magainin 1-induced leakage of entrapped calcein out of negatively-charged lipid vesicles. Biochim Biophys Acta (BBA) – Biomembr 1989;981:130–4. [59] Williams RW, Starman R, Taylor KMP, Gable K, Beeler T, Zasloff M, et al. Raman spectroscopy of synthetic antimicrobial frog peptides magainin 2a and PGLa. Biochemistry 1990;29:4490–6. [60] Bechinger B, Zasloff M, Opella SJ. Structure and orientation of the antibiotic peptide magainin in membranes by solid-state nuclear magnetic resonance spectroscopy. Protein Sci 1993;2:2077–84. [61] Boman HG, Wade D, Boman IA, Wåhlin B, Merrifield RB. Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett 1989;259:103–6. [62] Terwilliger TC, Eisenberg D. The structure of melittin. I. Structure determination and partial refinement. J Biol Chem 1982;257:6010–5. [63] Frey S, Tamm LK. Orientation of melittin in phospholipid bilayers. A polarized attenuated total reflection infrared study. Biophys J 1991;60:922–30. [64] Cornut I, Desbat B, Turlet JM, Dufourcq J. In situ study by polarization modulated Fourier transform infrared spectroscopy of the structure and orientation of lipids and amphipathic peptides at the air-water interface. Biophys J 1996;70:305–12. [65] Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 2003;3:710–20. [66] Falla TJ, Karunaratne DN, Hancock REW. Mode of action of the antimicrobial peptide indolicidin. J Biol Chem 1996;271:19298–303. 174 V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 [67] Rozek A, Friedrich CL, Hancock REW. Structure of the bovine antimicrobial peptide indolicidin bound to dodecylphosphocholine and sodium dodecyl sulfate micelles. Biochemistry 2000;39:15765–74. [68] Chan YR, Zanetti M, Gennaro R, Gallo RL. Anti-microbial activity and cell binding are controled by sequence determinants in the anti-microbial peptide PR-39. J Invest Dermatol 2001;116:230–5. [69] Nibbering PH, Ravensbergen E, Welling MM, van Berkel LA, van Berkel PHC, Pauwels EKJ, et al. Human lactoferrin and peptides derived from its N terminus are highly effective against infections with antibiotic-resistant bacteria. Infect Immun 2001;69:1469–76. [70] Dziarski R. Peptidoglycan recognition proteins (PGRPs). Mol Immunol 2004;40:877–86. [71] Garver LS, Wu J, Wu LP. The peptidoglycan recognition protein PGRP-SC1a is essential for Toll signaling and phagocytosis of Staphylococcus aureus in Drosophila. Proc Natl Acad Sci USA 2006;103:660–5. [72] Adlerova L, Bartoskova A, Faldyna M. Lactoferrin: a review. Vet Med 2008;53:457–68. [73] Chan DI, Prenner EJ, Vogel HJ. Tryptophan- and arginine-rich antimicrobial peptides: structures and mechanisms of action. Biochim Biophys Acta (BBA) – Biomembr 2006;1758:1184–202. [74] Yen C-C, Shen C-J, Hsu W-H, Chang Y-H, Lin H-T, Chen H-L, et al. Lactoferrin: an iron-binding antimicrobial protein against Escherichia coli; infection. BioMetals 2011;24:585–94. [75] Erdei J, Forsgren A, Naidu AS. Lactoferrin binds to porins OmpF and OmpC in Escherichia coli. Infect Immun 1994;62:1236–40. [76] Naidu SS, Svensson U, Kishore AR, Naidu AS. Relationship between antibacterial activity and porin binding of lactoferrin in Escherichia coli and Salmonella typhimurium. Antimicrob Agents Chemother 1993;37:240–5. [77] Sallmann FR, Baveye-Descamps S, Pattus F, Salmon V, Branza N, Spik G, et al. Porins OmpC and PhoE of Escherichia coli as specific cell-surface targets of human lactoferrin. J Biol Chem 1999;274:16107–14. [78] Wakabayashi H, Takase M, Tomita M. Lactoferricin derived from milk protein lactoferrin. Curr Pharm Des 2003;9:1277–87. [79] Strøm MB, Rekdal O, Svendsen JS. Antibacterial activity of 15-residue lactoferricin derivatives. J Pept Res 2000;56:265–74. [80] Dijkshoorn L, Brouwer CPJM, Bogaards SJP, Nemec A, van den Broek PJ, Nibbering PH. The synthetic N-terminal peptide of human lactoferrin, hLF(1– 11), is highly effective against experimental infection caused by multidrugresistant acinetobacter baumannii. Antimicrob Agents Chemother 2004;48:4919–21. [81] Azuma M, Del Carpio CA, Kojima T, Yokoyama I, Tajiri H, Yoshikawa K, et al. Antibacterial activity of multiple antigen peptides homologous to a loop region in human lactoferrin. J Pept Res 1999;54:237–41. [82] Flores-Villaseñor H, Canizalez-Román A, Reyes-Lopez M, Nazmi K, de la Garza M, Zazueta-Beltrán J, et al. Bactericidal effect of bovine lactoferrin, LFcin, LFampin and LFchimera on antibiotic-resistant Staphylococcus aureus and Escherichia coli. Biometals 2010;23:569–78. [83] Zweytick D, Tumer S, Blondelle SE, Lohner K. Membrane curvature stress and antibacterial activity of lactoferricin derivatives. Biochem Biophys Res Commun 2008;369:395–400. [84] Zweytick D, Pabst G, Abuja PM, Jilek A, Blondelle SE, Andrä J, et al. Influence of N-acylation of a peptide derived from human lactoferricin on membrane selectivity. Bioch Biophys Acta (BBA) – Biomembr 2006;1758:1426–35. [85] Bolscher JGM, Adão R, Nazmi K, van den Keybus PAM, van ‘t Hof W, Nieuw Amerongen AV, et al. Bactericidal activity of LFchimera is stronger and less sensitive to ionic strength than its constituent lactoferricin and lactoferrampin peptides. Biochimie 2009;91:123–32. [86] Leon-Sicairos N, Canizalez-Roman A, de la Garza M, Reyes-Lopez M, ZazuetaBeltran J, Nazmi K, et al. Bactericidal effect of lactoferrin and lactoferrin chimera against halophilic Vibrio parahaemolyticus. Biochimie 2009;91:133–40. [87] Lupetti A, Paulusma-Annema A, Senesi S, Campa M, van Dissel JT, Nibbering PH. Internal thiols and reactive oxygen species in candidacidal activity exerted by an N-terminal peptide of human lactoferrin. Antimicrob Agents Chemother 2002;46:1634–9. [88] Lohner K, Latal A, Degovics G, Garidel P. Packing characteristics of a model system mimicking cytoplasmic bacterial membranes. Chem Phys Lipids 2001;111:177–92. [89] Pozo Navas B, Lohner K, Deutsch G, Sevcsik E, Riske KA, Dimova R, et al. Composition dependence of vesicle morphology and mixing properties in a bacterial model membrane system. Biochim Biophys Acta (BBA) – Biomembr 2005;1716:40–8. [90] White GF, Racher KI, Lipski A, Hallett FR, Wood JM. Physical properties of liposomes and proteoliposomes prepared from Escherichia coli polar lipids. Biochim Biophys Acta (BBA) – Biomembr 2000;1468:175–86. [91] Murzyn K, Róg T, Pasenkiewicz-Gierula M. Phosphatidylethanolaminephosphatidylglycerol bilayer as a model of the inner bacterial membrane. Biophys J 2005;88:1091–103. [92] Zhao W, Róg T, Gurtovenko AA, Vattulainen I, Karttunen M. Role of phosphatidylglycerols in the stability of bacterial membranes. Biochimie 2008;90:930–8. [93] Epand RM, Rotem S, Mor A, Berno B, Epand RF. Bacterial membranes as predictors of antimicrobial potency. J Am Chem Soc 2008;130:14346–52. [94] Wydro P, Witkowska K. The interactions between phosphatidylglycerol and phosphatidylethanolamines in model bacterial membranes: The effect of the acyl chain length and saturation. Colloids Surf B: Biointerfaces 2009;72:32–9. [95] Teixeira V, Feio MJ, Rivas L, De la Torre BG, Andreu D, Coutinho A, et al. Influence of lysine Ne-trimethylation and lipid composition on the membrane activity of the cecropin A-melittin hybrid peptide CA(1–7)M(2– 9). J Phys Chem B 2010;114:16198–208. [96] Arouri A, Dathe M, Blume A. Peptide induced demixing in PG/PE lipid mixtures: a mechanism for the specificity of antimicrobial peptides towards bacterial membranes? Biochim Biophys Acta (BBA) – Biomembr 2008;1788:650–9. [97] Zhao H, Kinnunen PKJ. Binding of the antimicrobial peptide temporin L to liposomes assessed by Trp fluorescence. J Biol Chem 2002;277:25170–7. [98] Zhou C, Qi X, Li P, Chen WN, Mouad L, Chang MW, et al. High potency and broad-spectrum antimicrobial peptides synthesized via ring-opening polymerization of a-aminoacid-N-carboxyanhydrides. Biomacromolecules 2009;11:60–7. [99] Raghuraman H, Chattopadhyay A. Interaction of melittin with membrane cholesterol: a fluorescence approach. Biophys J 2004;87:2419–32. [100] Benachir T, Monette M, Grenier J, Lafleur M. Melittin-induced leakage from phosphatidylcholine vesicles is modulated by cholesterol: a property used for membrane targeting. Eur Biophys J 1997;25:201–10. [101] Suttmann H, Retz M, Paulsen F, Harder J, Zwergel U, Kamradt J, et al. Antimicrobial peptides of the Cecropin-family show potent antitumor activity against bladder cancer cells. BMC Urol 2008;8:5. [102] Lehmann J, Retz M, Sidhu SS, Suttmann H, Sell M, Paulsen F, et al. Antitumor activity of the antimicrobial peptide magainin II against bladder cancer cell lines. Eur Urol 2006;50:141–7. [103] Li YC, Park MJ, Ye S-K, Kim C-W, Kim Y-N. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol 2006;168:1107–18. [104] Andreu D, Rivas L. Animal antimicrobial peptides: an overview. Pept Sci 1998;47:415–33. [105] Abrunhosa F, Faria S, Gomes P, Tomaz I, Pessoa JC, Andreu D, et al. Interaction and lipid-induced conformation of two cecropin–melittin hybrid peptides depend on peptide and membrane composition. J Phys Chem B 2005;109:17311–9. [106] Bastos M, Bai G, Gomes P, Andreu D, Goormaghtigh E, Prieto M. Energetics and partition of two cecropin-melittin hybrid peptides to model membranes of different composition. Biophys J 2008;94:2128–41. [107] Kuchinka E, Seelig J. Interaction of melittin with phosphatidylcholine membranes. Binding isotherm and lipid head-group conformation. Biochemistry 1989;28:4216–21. [108] Belokoneva OS, Villegas E, Corzo G, Dai L, Nakajima T. The hemolytic activity of six arachnid cationic peptides is affected by the phosphatidylcholine-tosphingomyelin ratio in lipid bilayers. Biochim Biophys Acta (BBA) – Biomembr 2003;1617:22–30. [109] Melo MN, Castanho MARB. Omiganan interaction with bacterial membranes and cell wall models. Assigning a biological role to saturation. Biochim Biophys Acta (BBA) – Biomembr 2007;1768:1277–90. [110] Wieprecht T, Apostolov O, Beyermann M, Seelig J. Thermodynamics of the [alpha]-helix-coil transition of amphipathic peptides in a membrane environment: implications for the peptide-membrane binding equilibrium. J Mol Biol 1999;294:785–94. [111] White SH, Wimley WC. Membrane protein folding and stability: physical principles. Ann Rev Biophys Biomol Struct 1999;28:319–65. [112] Seelig J. Thermodynamics of lipid-peptide interactions. Biochim Biophys Acta (BBA) – Biomembr 2004;1666:40–50. [113] Toke O. Antimicrobial peptides: new candidates in the fight against bacterial infections. Pept Sci 2005;80:717–35. [114] Chen Y, Mant CT, Farmer SW, Hancock REW, Vasil ML, Hodges RS. Rational design of a-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J Biol Chem 2005;280:12316–29. [115] Oren Z, Shai Y. Selective lysis of bacteria but not mammalian cells by diastereomers of melittin: structure–function study. Biochemistry 1997;36:1826–35. [116] Shai Y, Oren Z. Diastereomers of cytolysins, a novel class of potent antibacterial peptides. J Biol Chem 1996;271:7305–8. [117] Dathe M, Schumann M, Wieprecht T, Winkler A, Beyermann M, Krause E, et al. Peptide helicity and membrane surface charge modulate the balance of electrostatic and hydrophobic interactions with lipid bilayers and biological membranes. Biochemistry 1996;35:12612–22. [118] Bessalle R, Haas H, Goria A, Shalit I, Fridkin M. Augmentation of the antibacterial activity of magainin by positive-charge chain extension. Antimicrob Agents Chemother 1992;36:313–7. [119] Marshall SH, Arenas G. Antimicrobial peptides: a natural alternative to chemical antibiotics and a potential for applied biotechnology. Electronic J Biotechnol 2003;6:271–84. [120] Harris F, Dennison SR, Phoenix DA. Anionic antimicrobial peptides from eukaryotic organisms. Curr Protein Pept Sci 2009;10:585–606. [121] Eisenberg D, Weiss RM, Terwilliger TC. The hydrophobic moment detects periodicity in protein hydrophobicity. Proc Natl Acad Sci USA 1984;81: 140–4. [122] Wieprecht T, Dathe M, Krause E, Beyermann M, Maloy WL, MacDonald DL, et al. Modulation of membrane activity of amphipathic, antibacterial peptides by slight modifications of the hydrophobic moment. FEBS Lett 1997;417:135–40. [123] Kondejewski L, McInnes C, Jelokhani-Niaraki M, Farmer S, Kay C, Sykes B, et al. Modulation of specificity in cyclic antimicrobial peptides by V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 [124] [125] [126] [127] [128] [129] [130] [131] [132] [133] [134] [135] [136] [137] [138] [139] [140] [141] [142] [143] [144] [145] [146] [147] [148] [149] [150] amphipathicity. In: Fields GB, Tam JP, Barany G, editors. Peptides for the New Millennium. Netherlands: Springer Netherlands; 2002. p. 752–3. Stark M, Liu L-P, Deber CM. Cationic hydrophobic peptides with antimicrobial activity. Antimicrob Agents Chemother 2002;46:3585–90. Chen Y, Guarnieri MT, Vasil AI, Vasil ML, Mant CT, Hodges RS. Role of peptide hydrophobicity in the mechanism of action of [alpha]-helical antimicrobial peptides. Antimicrob Agents Chemother 2007;51:1398–406. Chou H-T, Kuo T-Y, Chiang J-C, Pei M-J, Yang W-T, Yu H-C, et al. Design and synthesis of cationic antimicrobial peptides with improved activity and selectivity against Vibrio spp. Int J Antimicrob Agents 2008;32:130–8. Kondejewski LH, Lee DL, Jelokhani-Niaraki M, Farmer SW, Hancock REW, Hodges RS. Optimization of microbial specificity in cyclic peptides by modulation of hydrophobicity within a defined structural framework. J Biol Chem 2002;277:67–74. Prenner EJ, Kiricsi M, Jelokhani-Niaraki M, Lewis RNAH, Hodges RS, McElhaney RN. Structure–activity relationships of diastereomeric lysine ring size analogs of the antimicrobial peptide gramicidin S. J Biol Chem 2005;280:2002–11. Dathe M, Wieprecht T. Structural features of helical antimicrobial peptides: their potential to modulate activity on model membranes and biological cells. Biochim Biophys Acta (BBA) – Biomembr 1999;1462:71–87. Uematsu N, Matsuzaki K. Polar angle as a determinant of amphipathic [alpha]-helix-lipid interactions: a model peptide study. Biophys J 2000;79:2075–83. Dathe M, Wieprecht T, Nikolenko H, Handel L, Maloy WL, MacDonald DL, et al. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett 1997;403:208–12. Powers J-PS, Martin MM, Goosney DL, Hancock REW. The antimicrobial peptide polyphemusin localizes to the cytoplasm of Escherichia coli following treatment. Antimicrob Agents Chemother 2006;50:1522–4. Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, et al. [Beta]defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 1999;286:525–8. Oehlke J, Lorenz D, Wiesner B, Bienert M. Studies on the cellular uptake of substance P and lysine-rich, KLA-derived model peptides. J Mol Recognit 2005;18:50–9. Meincken M, Holroyd DL, Rautenbach M. Atomic force microscopy study of the effect of antimicrobial peptides on the cell envelope of Escherichia coli. Antimicrob Agents Chemother 2005;49:4085–92. Grasso D, Milardi D, Rosa CL, Impellizzeri G, Pappalardo G. The interaction of a peptide with a scrambled hydrophobic/hydrophilic sequence (Pro-Asp-AlaAsp-Ala-His-Ala-His-Ala-His-Ala-Ala-Ala-His-Gly) (PADH) with DPPC model membranes: a DSC study. Thermochim Acta 2002;390:73–8. Epand RM, Epand RF. Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochim Biophys Acta (BBA) – Biomembr 2009;1788:289–94. Hancock REW. Peptide antibiotics. Lancet 1997;349:418–22. Hancock REW, Bell A. Antibiotic uptake into gram-negative bacteria. Eur J Clin Microbiol Infect Dis 1988;7:713–20. Li A, Lee PY, Ho B, Ding JL, Lim CT. Atomic force microscopy study of the antimicrobial action of Sushi peptides on Gram negative bacteria. Biochim Biophys Acta (BBA) – Biomembr 2007;1768:411–8. Breukink E, Wiedemann I, Kraaij Cv, Kuipers OP, Sahl H-G, de Kruijff B. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 1999;286:2361–4. Hsu S-TD, Breukink E, Tischenko E, Lutters MAG, de Kruijff B, Kaptein R, et al. The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat Struct Mol Biol 2004;11:963–7. Dalet K, Cenatiempo Y, Cossart P, Hechard Y. A {sigma}54-dependent PTS permease of the mannose family is responsible for sensitivity of Listeria monocytogenes to mesentericin Y105. Microbiology 2001;147:3263–9. Destoumieux-Garzón D, Duquesne S, Peduzzi J, Goulard C, Desmadril M, Letellier L, et al. The iron-siderophore transporter FhuA is the receptor for the antimicrobial peptide microcin J25: role of the microcin Val11-Pro16 [beta]hairpin region in the recognition mechanism. Biochem J 2005;389:869–76. Ludtke SJ, He K, Wu Y, Huang HW. Cooperative membrane insertion of magainin correlated with its cytolytic activity. Biochim Biophys Acta (BBA) – Biomembr 1994;1190:181–4. Heller WT, He K, Ludtke SJ, Harroun TA, Huang HW. Effect of changing the size of lipid headgroup on peptide insertion into membranes. Biophys J 1997;73:239–44. Strandberg E, Wadhwani P, Tremouilhac P, Dürr UHN, Ulrich AS. Solid-state NMR analysis of the PGLa peptide orientation in DMPC bilayers: structural fidelity of 2H-labels versus high sensitivity of 19F-NMR. Biophys J 2006;90:1676–86. Mason AJ, Marquette A, Bechinger B. Zwitterionic phospholipids and sterols modulate antimicrobial peptide-induced membrane destabilization. Biophys J 2007;93:4289–99. Bechinger B. Rationalizing the membrane interactions of cationic amphipathic antimicrobial peptides by their molecular shape. Curr Opin Colloid Interf Sci 2009;14:349–55. Lee M-T, Hung W-C, Chen F-Y, Huang HW. Many-body effect of antimicrobial peptides: on the correlation between lipid’s spontaneous curvature and pore formation. Biophys J 2005;89:4006–16. 175 [151] Klocek G, Schulthess T, Shai Y, Seelig J. Thermodynamics of melittin binding to lipid bilayers. Aggregation and pore formation. Biochemistry 2009;48:2586–96. [152] Seelig J, Ganz P. Nonclassical hydrophobic effect in membrane binding equilibria. Biochemistry 2002;30:9354–9. [153] Kaiser ET, Kézdy FJ. Secondary structures of proteins and peptides in amphiphilic environments. A review. Proc Natl Acad Sci USA 1983;80:1137–43. [154] Wimley WC, White SH. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat Struct Mol Biol 1996;3:842–8. [155] Ooi T, Oobatake M. Prediction of the thermodynamics of protein unfolding: the helix-coil transition of poly(L-alanine). Proc Natl Acad Sci USA 1991;88:2859–63. [156] Scholtz JM, Marqusee S, Baldwin RL, York EJ, Stewart JM, Santoro M, et al. Calorimetric determination of the enthalpy change for the [alpha]-helix to coil transition of an alanine peptide in water. Proc Natl Acad Sci USA 1991;88:2854–8. [157] Scholtz JM, Qian H, York EJ, Stewart JM, Baldwin RL. Parameters of helix–coil transition theory for alanine-based peptides of varying chain lengths in water. Biopolymers 1991;31:1463–70. [158] Bastos M, Pease JHB, Wemmer DE, Murphy KP, Connelly PR. Thermodynamics of the helix-coil transition: Binding of S15 and a hybrid sequence, disulfide stabilized peptide to the S-protein. Proteins: Struct Funct Bioinform 2001;42:523–30. [159] White SH, Wimley WC. Hydrophobic interactions of peptides with membrane interfaces. Biochim Biophys Acta (BBA) – Rev Biomembr 1998;1376:339–52. [160] Tam JP, Lu Y-A, Yang J-L. Marked increase in membranolytic selectivity of novel cyclic tachyplesins constrained with an antiparallel two-[beta] strand cystine knot framework. Biochem Biophys Res Commun 2000;267:783–90. [161] Christensen B, Fink J, Merrifield RB, Mauzerall D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc Natl Acad Sci USA 1988;85:5072–6. [162] Mazzuca C, Stella L, Venanzi M, Formaggio F, Toniolo C, Pispisa B. Mechanism of membrane activity of the antibiotic trichogin GA IV: a two-state transition controlled by peptide concentration. Biophys J 2005;88:3411–21. [163] Mahalka AK, Kinnunen PKJ. Binding of amphipathic [alpha]-helical antimicrobial peptides to lipid membranes: lessons from temporins B and L. Biochim Biophys Acta (BBA) – Biomembr 2009;1788:1600–9. [164] Zhao H, Sood R, Jutila A, Bose S, Fimland G, Nissen-Meyer J, et al. Interaction of the antimicrobial peptide pheromone Plantaricin A with model membranes: Implications for a novel mechanism of action. Biochim Biophys Acta (BBA) – Biomembr 2006;1758:1461–74. [165] Vedovato N, Rispoli G. A novel technique to study pore-forming peptides in a natural membrane. Eur Biophys J 2007;36:771–8. [166] Spellberg B, Powers John H, Brass Eric P, Miller Loren G, Edwards J, John E. Trends in antimicrobial drug development: implications for the future. Clin Infect Dis 2004;38:1279–86. [167] Eisenberg M, Hall JE, Mead CA. The nature of the voltage-dependent conductance induced by alamethicin in black lipid membranes. J Membr Biol 1973;14:143–76. [168] Shenkarev ZO, Balashova TA, Efremov RG, Yakimenko ZA, Ovchinnikova TV, Raap J, et al. Spatial structure of zervamicin IIB bound to DPC micelles: implications for voltage-gating. Biophys J 2002;82:762–71. [169] Shai Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by [alpha]-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta (BBA) – Biomembr 1999;1462:55–70. [170] Yamaguchi S, Huster D, Waring A, Lehrer RI, Kearney W, Tack BF, et al. Orientation and dynamics of an antimicrobial peptide in the lipid bilayer by solid-state NMR spectroscopy. Biophys J 2001;81:2203–14. [171] Papo N, Shai Y. Exploring peptide membrane interaction using surface plasmon resonance: differentiation between pore formation versus membrane disruption by lytic peptides. Biochemistry 2002;42:458–66. [172] Sitaram N, Nagaraj R. Interaction of antimicrobial peptides with biological and model membranes: structural and charge requirements for activity. Biochim Biophys Acta (BBA) – Biomembr 1999;1462:29–54. [173] Sengupta D, Leontiadou H, Mark AE, Marrink S-J. Toroidal pores formed by antimicrobial peptides show significant disorder. Biochim Biophys Acta (BBA) – Biomembr 2008;1778:2308–17. [174] Silvestro L, Gupta K, Weiser JN, Axelsen PH. The concentration-dependent membrane activity of cecropin A. Biochemistry 1997;36:11452–60. [175] Wu M, Maier E, Benz R, Hancock REW. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 1999;38:7235–42. [176] Miteva M, Andersson M, Karshikoff A, Otting G. Molecular electroporation: a unifying concept for the description of membrane pore formation by antibacterial peptides, exemplified with NK-lysin. FEBS Lett 1999;462:155–8. [177] Karshikov A, Berendes R, Burger A, Cavalié A, Lux H-D, Huber R. Annexin V membrane interaction: an electrostatic potential study. Eur Biophys J 1992;20:337–44. [178] Jean-Marie R, Erik G, Homblé F, Mats A, Edvards L, Gottfried O. Lipid membrane binding of NK-lysin. FEBS Lett 1998;425:341–4. [179] Pokorny A, Birkbeck TH, Almeida PFF. Mechanism and kinetics of d-lysin interaction with phospholipid vesicles. Biochemistry 2002;41:11044–56. 176 V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 [180] Pokorny A, Almeida PFF. Kinetics of dye efflux and lipid flip-flop induced by dlysin in phosphatidylcholine vesicles and the mechanism of graded release by amphipathic, a-helical peptides. Biochemistry 2004;43:8846–57. [181] Pokorny A, Almeida PFF. Permeabilization of raft-containing lipid vesicles by d-lysin: a mechanism for cell sensitivity to cytotoxic peptides. Biochemistry 2005;44:9538–44. [182] Haney EF, Nathoo S, Vogel HJ, Prenner EJ. Induction of non-lamellar lipid phases by antimicrobial peptides: a potential link to mode of action. Chem Phys Lipids 2010;163:82–93. [183] Epand RF, Maloy L, Ramamoorthy A, Epand RM. Amphipathic helical cationic antimicrobial peptides promote rapid formation of crystalline states in the presence of phosphatidylglycerol: lipid clustering in anionic membranes. Biophys J 2010;98:2564–73. [184] Fernández-Reyes Ma, Díaz D, de la Torre BG, Cabrales-Rico A, Vallès-Miret M, Jiménez-Barbero Js, et al. Lysine Ne-trimethylation, a tool for improving the selectivity of antimicrobial peptides. J Med Chem 2010;53:5587–96. [185] Epand RF, Wang G, Berno B, Epand RM. Lipid segregation explains selective toxicity of a series of fragments derived from the human cathelicidin LL-37. Antimicrob Agents Chemother 2009;53:3705–14. [186] Den Hertog AL, Van Marle J, Veerman ECI, Valentijn-Benz M, Nazmi K, Kalay H, et al. The human cathelicidin peptide LL-37 and truncated variants induce segregation of lipids and proteins in the plasma membrane of Candida albicans. J Biol Chem 2006;387:1495–502. [187] Iwamoto K, Kobayashi S, Fukuda R, Umeda M, Kobayashi T, Ohta A. Local exposure of phosphatidylethanolamine on the yeast plasma membrane is implicated in cell polarity. Genes Cells 2004;9:891–903. [188] Nishibori A, Kusaka J, Hara H, Umeda M, Matsumoto K. Phosphatidylethanolamine domains and localization of phospholipid synthases in Bacillus subtilis membranes. J Bacteriol 2005;187:2163–74. [189] Emoto K, Umeda M. An essential role for a membrane lipid in cytokinesis: regulation of contractile ring disassembly by redistribution of phosphatidylethanolamine. J Cell Biol 2000;149:1215–24. [190] Huttner WB, Zimmerberg J. Implications of lipid microdomains for membrane curvature, budding and fission: commentary. Curr Opin Cell Biol 2001;13:478–84. [191] Matsumoto K, Kusaka J, Nishibori A, Hara H. Lipid domains in bacterial membranes. Mol Microbiol 2006;61:1110–7. [192] Yamamoto N, Tamura A. Designed low amphipathic peptides with [alpha]helical propensity exhibiting antimicrobial activity via a lipid domain formation mechanism. Peptides 2010;5:794–805. [193] Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature 2004;430:631–9. [194] Norris V, Fishov I. Hypothesis: membrane domains and hyperstructures control bacterial division. Biochimie 2001;83:91–7. [195] Woldringh CL. The role of co-transcriptional translation and protein translocation (transertion) in bacterial chromosome segregation. Mol Microbiol 2002;45:17–29. [196] Rossjohn J, Feil SC, McKinstry WJ, Tweten RK, Parker MW. Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell 1997;89:685–92. [197] Zhao H, Tuominen EKJ, Kinnunen PKJ. Formation of amyloid fibers triggered by phosphatidylserine-containing membranes. Biochemistry 2004;43:10302–7. [198] Kirk GL, Gruner SM, Stein DL. A thermodynamic model of the lamellar to inverse hexagonal phase transition of lipid membrane-water systems. Biochemistry 1984;23:1093–102. [199] Epand RM. Lipid polymorphism and protein-lipid interactions. Biochim Biophys Acta (BBA) – Rev Biomembr 1998;1376:353–68. [200] Bastos M, Silva T, Teixeira V, Nazmi K, Bolscher JGM, Funari SS, Uhríková D. Lactoferrin derived antimicrobial peptide induces a micellar cubic phase in a model membrane system. Biophys J 2011 (just accepted). [201] Luzzati V. Biological significance of lipid polymorphism: the cubic phases. Curr Opin Struct Biol 1997;7:661–8. [202] Deatherage JF, Taylor KA, Amos LA, Moody MF. Three-dimensional arrangement of the cell wall protein of Sulfolobus acidocaldarius. J Mol Biol 1983;167:823–48. [203] Epand RM, Epand RF. Modulation of membrane curvature by peptides. Pept Sci 2000;55:358–63. [204] Epand RM. Chapter 6 modulation of lipid polymorphism by peptides. In: Epand RM, editor. Current topics in membranes and transport. Academic Press, Inc.; 1997. p. 237–52. [205] Sevcsik E, Pabst G, Jilek A, Lohner K. How lipids influence the mode of action of membrane-active peptides. Biochim Biophys Acta (BBA) – Biomembr 2007;1768:2586–95. [206] Powers J-PS, Tan A, Ramamoorthy A, Hancock REW. Solution structure and interaction of the antimicrobial polyphemusins with lipid membranes. Biochemistry 2005;44:15504–13. [207] Prenner EJ, Lewis RNAH, McElhaney RN. The interaction of the antimicrobial peptide gramicidin S with lipid bilayer model and biological membranes. Biochim Biophys Acta (BBA) – Biomembr 1999;1462:201–21. [208] Prenner EJ, Lewis RNAH, Neuman KC, Gruner SM, Kondejewski LH, Hodges RS, et al. Nonlamellar phases induced by the interaction of gramicidin S with lipid bilayers. A possible relationship to membrane-disrupting activity. Biochemistry 1997;36:7906–16. [209] Fontell K. Cubic phases in surfactant and surfactant-like lipid systems. Colloid Polym Sci 1990;268:264–85. [210] Staudegger E, Prenner EJ, Kriechbaum M, Degovics G, Lewis RNAH, McElhaney RN, et al. X-ray studies on the interaction of the antimicrobial peptide gramicidin S with microbial lipid extracts: evidence for cubic phase formation. Biochim Biophys Acta (BBA) – Biomembr 2000;1468: 213–30. [211] Hickel A, Danner-Pongratz S, Amenitsch H, Degovics G, Rappolt M, Lohner K, et al. Influence of antimicrobial peptides on the formation of nonlamellar lipid mesophases. Biochim Biophys Acta (BBA) – Biomembr 2008;1778: 2325–33. [212] El Jastimi R, Lafleur M. Nisin promotes the formation of non-lamellar inverted phases in unsaturated phosphatidylethanolamines. Biochim Biophys Acta (BBA) – Biomembr 1999;1418:97–105. [213] Keller SL, Gruner SM, Gawrisch K. Small concentrations of alamethicin induce a cubic phase in bulk phosphatidylethanolamine mixtures. Biochim Biophys Acta (BBA) – Biomembr 1996;1278:241–6. [214] Hurley JH, Misra S. Signaling and subcellular targeting by membrane-binding domains. Ann Rev Biophys Biomol Struct 2000;29:49–79. [215] Martínez J, Vögler O, Casas J, Barceló F, Alemany R, Prades J, et al. Membrane structure modulation, protein kinase ca activation, and anticancer activity of minerval. Mol Pharmacol 2005;67:531–40. [216] Skerlavaj B, Scocchi M, Tossi A, Romeo D, Gennaro R. A synthetic approach for SAR study of the Pro- and Arg-rich Bactenecin Bac7. In: Epton R, editor. Innovation and perspectives in solid phase synthesis. Birmingham, UK: Mayflower Scientific Ltd; 1998. p. 395–8. [217] Zhao H, Kinnunen PKJ. Modulation of the activity of secretory phospholipase A2 by antimicrobial peptides. Antimicrob Agents Chemother 2003;47:965–71. [218] Park CB, Kim HS, Kim SC. Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem Biophys Res Commun 1998;244:253–7. [219] Park CB, Yi K-S, Matsuzaki K, Kim MS, Kim SC. Structure–activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: the proline hinge is responsible for the cell-penetrating ability of buforin II. Proc Natl Acad Sci USA 2000;97:8245–50. [220] Shi J, Ross C, Chengappa M, Sylte M, McVey D, Blecha F. Antibacterial activity of a synthetic peptide (PR-26) derived from PR-39, a proline-arginine-rich neutrophil antimicrobial peptide. Antimicrob Agents Chemother 1996;40:115–21. [221] Su LY, Willner DL, Segall AM. An antimicrobial peptide that targets DNA repair intermediates in vitro inhibits Salmonella growth within murine macrophages. Antimicrob Agents Chemother 2010;54:1888–99. [222] Pierrat OA, Maxwell A. The action of the bacterial toxin microcin B17. J Biol Chem 2003;278:35016–23. [223] Patrzykat A, Friedrich CL, Zhang L, Mendoza V, Hancock REW. Sublethal concentrations of pleurocidin-derived antimicrobial peptides inhibit macromolecular synthesis in Escherichia coli. Antimicrob Agents Chemother 2002;46:605–14. [224] Gifford J, Hunter H, Vogel H. Lactoferricin. Cell Mol Life Sci 2005;62:2588–98. [225] Kragol G, Lovas S, Varadi G, Condie BA, Hoffmann R, Otvos L. The antibacterial peptide pyrrhocoricin inhibits the ATPase actions of DnaK and prevents chaperone-assisted protein folding. Biochemistry 2001;40:3016–26. [226] Lupetti A, Paulusma-Annema A, Welling MM, Senesi S, van Dissel JT, Nibbering PH. Candidacidal activities of human lactoferrin peptides derived from the N terminus. Antimicrob Agents Chemother 2000;44:3257–63. [227] Mader JS, Richardson A, Salsman J, Top D, de Antueno R, Duncan R, et al. Bovine lactoferricin causes apoptosis in Jurkat T-leukemia cells by sequential permeabilization of the cell membrane and targeting of mitochondria. Exp Cell Res 2007;313:2634–50. [228] Basselin M, Robert-Gero M. Alterations in membrane fluidity, lipid metabolism, mitochondrial activity, and lipophosphoglycan expression in pentamidine-resistant Leishmania. Parasitol Res 1998;84:78–83. [229] Dorrer E, Teuber M. Induction of polymyxin resistance in Pseudomonas fluorescens by phosphate limitation. Arch Microbiol 1977;114:87–9. [230] Peschel A, Jack RW, Otto M, Collins LV, Staubitz P, Nicholson G, et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor Mprf is based on modification of membrane lipids with l-lysine. J Exp Med 2001;193: 1067–76. [231] Abachin E, Poyart C, Pellegrini E, Milohanic E, Fiedler F, Berche P, et al. Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol Microbiol 2002;43:1–14. [232] Poyart C, Pellegrini E, Marceau M, Baptista M, Jaubert F, Lamy M-C, et al. Attenuated virulence of Streptococcus agalactiae deficient in D-alanyllipoteichoic acid is due to an increased susceptibility to defensins and phagocytic cells. Mol Microbiol 2003;49:1615–25. [233] Friedrich C, Scott MG, Karunaratne N, Yan H, Hancock REW. Salt-resistant [alpha]-helical cationic antimicrobial peptides. Antimicrob Agents Chemother 1999;43:1542–8. [234] Ernst RK, Guina T, Miller SI. Salmonella typhimurium outer membrane remodeling: role in resistance to host innate immunity. Microb Infect 2001;3:1327–34. [235] Lysenko ES, Gould J, Bals R, Wilson JM, Weiser JN. Bacterial phosphorylcholine decreases susceptibility to the antimicrobial peptide LL37/hCAP18 expressed in the upper respiratory tract. Infect Immun 2000;68:1664–71. V. Teixeira et al. / Progress in Lipid Research 51 (2012) 149–177 [236] Lehrer RI, Ganz T. Endogenous vertebrate antibiotics: defensins, protegrins, and other cysteine-rich antimicrobial peptides. Ann NY Acad Sci 1996;797:228–39. [237] Yeaman MR, Bayer AS, Koo SP, Foss W, Sullam PM. Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J Clin Invest 1998;101:178–87. [238] Hol WGJ, Halie LM, Sander C. Dipoles of the [alpha]-helix and [beta]-sheet: their role in protein folding. Nature 1981;294:532–6. [239] Gyurko C, Lendenmann U, Troxler RF, Oppenheim FG. Candida albicans mutants deficient in respiration are resistant to the small cationic salivary antimicrobial peptide histatin 5. Antimicrob Agents Chemother 2000;44:348–54. [240] Hong RW, Shchepetov M, Weiser JN, Axelsen PH. Transcriptional profile of the Escherichia coli response to the antimicrobial insect peptide cecropin A. Antimicrob Agents Chemother 2003;47:1–6. [241] Groisman E, Kayser J, Soncini F. Regulation of polymyxin resistance and adaptation to low-Mg2+ environments. J Bacteriol 1997;179:7040–5. [242] Pilonieta MC, Erickson KD, Ernst RK, Detweiler CS. A protein important for antimicrobial peptide resistance, YdeI/OmdA, is in the periplasm and interacts with OmpD/NmpC. J Bacteriol 2009;191:7243–52. [243] Guina T, Yi EC, Wang H, Hackett M, Miller SI. A PhoP-regulated outer membrane protease of Salmonella enterica serovar typhimurium promotes resistance to [alpha]-helical antimicrobial peptides. J Bacteriol 2000;182:4077–86. [244] Ramu P, Tanskanen R, Holmberg M, Lähteenmäki K, Korhonen TK, Meri S. The surface protease PgtE of Salmonella enterica affects complement activity by proteolytically cleaving C3b, C4b and C5. FEBS Lett 2007;581:1716–20. [245] Ulvatne H, Haukland HH, Samuelsen O, Kramer M, Vorland LH. Proteases in Escherichia coli and Staphylococcus aureus confer reduced susceptibility to lactoferricin B. J Antimicrob Chemother 2002;50:461–7. [246] Ösapay K, Tran D, Ladokhin AS, White SH, Henschen AH, Selsted ME. Formation and characterization of a single Trp-Trp cross-link in indolicidin that confers protease stability without altering antimicrobial activity. J Biol Chem 2000;275:12017–22. [247] Shafer WM, Qu X-D, Waring AJ, Lehrer RI. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci USA 1998;95:1829–33. [248] Bengoechea JA, Skurnik M. Temperature-regulated efflux pump/potassium antiporter system mediates resistance to cationic antimicrobial peptides in Yersinia. Mol Microbiol 2000;37:67–80. [249] Kupferwasser LI, Skurray RA, Brown MH, Firth N, Yeaman MR, Bayer AS. Plasmid-mediated resistance to thrombin-induced platelet microbicidal protein in staphylococci: role of the qacA locus. Antimicrob Agents Chemother 1999;43:2395–9. [250] Bina XR, Provenzano D, Nguyen N, Bina JE. Vibrio cholerae RND family efflux systems are required for antimicrobial resistance, optimal virulence factor production, and colonization of the infant mouse small intestine. Infect Immun 2008;76:3595–605. [251] Wizemann TM, Adamou JE. S.L. Adhesins as targets for vaccine development. Emerg Infect Dis 1999;5:395–403. [252] Matsuzaki K, Mitani Y, Akada K-y, Murase O, Yoneyama S, Zasloff M, et al. Mechanism of synergism between antimicrobial peptides magainin 2 and PGLa. Biochemistry 1998;37:15144–53. [253] Giacometti A, Cirioni O, Kamysz W, D’Amato G, Silvestri C, Del Prete MS, et al. Comparative activities of cecropin A, melittin, and cecropin A-melittin peptide CA(1–7)M(2–9)NH2 against multidrug-resistant nosocomial isolates of Acinetobacter baumannii. Peptides 2003;24:1315–8. [254] Papo N, Shai Y. Host defense peptides as new weapons in cancer treatment. Cell Mol Life Sci 2005;62:784–90. [255] van der Velden W, van Iersel T, Blijlevens N, Donnelly JP. Safety and tolerability of the antimicrobial peptide human lactoferrin 1-11 (hLF1-11). BMC Med 2009;7:44. [256] Levin M, Quint PA, Goldstein B, Barton P, Bradley JS, Shemie SD, et al. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: a randomised trial. Lancet 2000;356:961–7. 177 [257] Herce HD, Garcia AE. Molecular dynamics simulations suggest a mechanism for translocation of the HIV-1 TAT peptide across lipid membranes. Proc Natl Acad Sci 2007;104:20805–10. [258] Tresset G. The multiple faces of self-assembled lipidic systems. PMC Biophys 2009;2:3. [259] Tardieu A, Luzzati V. A novel cubic phase-A cage-like network of rods with enclosed spherical micelles. Biochim Biophys Acta (BBA) – Biomembr 1970;219:11–7. [260] Fontell K, Fox KK, Hansson E. On the structure of the cubic phase I, in some lipid-water systems. Mol Cryst Liquid Cryst Lett 1985;1:9–17. [261] Charvolin J, Sadoc JF. Periodic systems of frustrated fluid films and «micellar» cubic structures in liquid crystals. J Phys France 1988;49:521–6. [262] Ratledge C, Wilkinson E SG. Microbial lipids, 3rd ed., vol. 1. London: Academic Press; 1988. [263] Morein S, Andersson A-S, Rilfors L, Lindblom G. Wild-type Escherichia coli cells regulate the membrane lipid composition in a window between gel and non-lamellar structures. J Biol Chem 1996;271:6801–9. [264] Verkleij AJ, Zwaal RFA, Roelofsen B, Comfurius P, Kastelijn D, van Deenen LLM. The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim Biophys Acta (BBA) – Biomembr 1973;323:178–93. [265] Fischer W. Lipoteichoic acid and lipids in the membrane of Staphylococcus aureus. Med Microbiol Immunol 1994;183:61–76. [266] Yonezawa A, Kuwahara J, Fujii N, Sugiura Y. Binding of tachyplesin I to DNA revealed by footprinting analysis: significant contribution of secondary structure to DNA binding and implication for biological action. Biochemistry 1992;31:2998–3004. [267] Zhang J, Wu X, Zhang S-Q. Antifungal mechanism of antibacterial peptide, ABP-CM4, from Bombyx mori against Aspergillus niger. Biotechnol Lett 2008;30:2157–63. [268] Lan Y, Ye Y, Kozlowska J, Lam JKW, Drake AF, Mason AJ. Structural contributions to the intracellular targeting strategies of antimicrobial peptides. Biochim Biophys Acta (BBA) – Biomembr 2010;1798:1934–43. [269] Lehrer RI, Barton A, Daher KA, Harwig SS, Ganz T, Selsted ME. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Invest 1989;84:553–61. [270] Boman HG, Agerberth B, Boman A. Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine. Infect Immun 1993;61:2978–84. [271] Subbalakshmi C, Sitaram N. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol Lett 1998;160:91–6. [272] Carrasco L, Vázquez D, Hernández-Lucas C, Carbonero P, García-Olmedo F. Thionins: plant peptides that modify membrane permeability in cultured mammalian cells. Eur J Biochem 1981;116:185–9. [273] Salomon RA, Farias RN. Microcin 25, a novel antimicrobial peptide produced by Escherichia coli. J Bacteriol 1992;174:7428–35. [274] Johnson TC, Wada K, Buchanan BB, Holmgren A. Reduction of purothionin by the wheat seed thioredoxin system. Plant Physiol 1987;85:446–51. [275] Scocchi M, Lüthy C, Decarli P, Mignogna G, Christen P, Gennaro R. The proline-rich antibacterial peptide Bac7 binds to and inhibits in vitro the molecular chaperone DnaK. Int J Pept Res Ther 2009;15:147–55. [276] Schneider T, Kruse T, Wimmer R, Wiedemann I, Sass V, Pag U, et al. Plectasin, a fungal defensin, targets the bacterial cell wall precursor lipid II. Science 2010;328:1168–72. [277] Brotz H, Bierbaum G, Leopold K, Reynolds PE, Sahl H-G. The lantibiotic mersacidin inhibits peptidoglycan synthesis by targeting lipid II. Antimicrob Agents Chemother 1998;42:154–60. [278] Destoumieux D, Munoz M, Bulet P, Bachère E. Penaeidins, a family of antimicrobial peptides from penaeid shrimp (Crustacea, Decapoda). Cell Mol Life Sci 2000;57:1260–71. [279] Risso A, Braidot E, Sordano MC, Vianello A, Macri F, Skerlavaj B, et al. BMAP28, an antibiotic peptide of innate immunity, induces cell death through opening of the mitochondrial permeability transition pore. Mol Cell Biol 2002;22:1926–35. [280] Delgado M, Anderson P, Garcia-Salcedo JA, Caro M, Gonzalez-Rey E. Neuropeptides kill African trypanosomes by targeting intracellular compartments and inducing autophagic-like cell death. Cell Death Differ 2008;16:406–16.