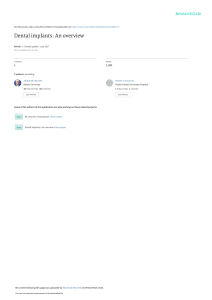
Review Article Performance of conical abutment (Morse Taper) connection implants: A systematic review Christian M. Schmitt,1 Getulio Nogueira-Filho,2 Howard C. Tenenbaum,3 Jim Yuan Lai,3 € ring,1,2 Jo € rg Nonhoff4 Carlos Brito,2 Hendrik Do 1 Department of Oral and Maxillofacial Surgery, University of Erlangen-Nuremberg, Erlangen, Germany Department of Preventive Dentistry, University of Toronto, Toronto, Ontario, Canada 3 Department of Periodontology, University of Toronto, Toronto, Ontario, Canada 4 Clinical Research DENTSPLY Friadent, Mannheim, Germany 2 Received 7 January 2013; revised 20 February 2013; accepted 20 February 2013 Published online 9 May 2013 in Wiley Online Library (wileyonlinelibrary.com). 10.1002/jbm.a.34709 Abstract: In this systematic review, we aimed to compare conical versus nonconical implant–abutment connection systems in terms of their in vitro and in vivo performances. An electronic search was performed using PubMed, Embase, and Medline databases with the logical operators: “dental implant” AND “dental abutment” AND (“conical” OR “taper” OR “cone”). Names of the most common conical implant– abutment connection systems were used as additional key words to detect further data. The search was limited to articles published up to November 2012. Recent publications were also searched manually in order to find any relevant studies that might have been missed using the search criteria noted above. Fifty-two studies met the inclusion criteria and were included in this systematic review. As the data and methods, as well as types of implants used was so heterogeneous, this mitigated against the performance of meta-analysis. In vitro studies indicated that conical and nonconical abutments showed sufficient resistance to maximal bending forces and fatigue loading. However, conical abutments showed superiority in terms of seal performance, microgap formation, torque maintenance, and abutment stability. In vivo studies (human and animal) indicated that conical and nonconical systems are comparable in terms of implant success and survival rates with less marginal bone loss around conical connection implants in most cases. This review indicates that implant systems using a conical implant–abutment connection, provides better results in terms of abutment fit, stability, and seal performance. These design features could lead to improvements over time versus nonconical connecC 2013 Wiley Periodicals, Inc. J Biomed Mater Res tion systems. V Part A: 102A: 552–574, 2014. Key Words: systematic review, dental implant, dental abutment, Morse Taper, implant–abutment connection € ring H, Nonhoff J. 2014. Performance of How to cite this article: Schmitt CM, Nogueira-Filho G, Tenenbaum HC, Lai JY, Brito C, Do conical abutment (Morse Taper) connection implants: A systematic review. J Biomed Mater Res Part A 2014:102A:552–574. INTRODUCTION Implant systems differ in terms of the geometry of the implant–abutment interface with particular differences between both conical and nonconical connection systems (indexed external or internal connections). The implant– abutment connection represents the weakest point of dental endosseous implant fixtures, as it must resist maximal and permanent masticatory forces as well as penetration by bacteria. The formation of a marginal gap between the implant and abutment might lead to increased loss of marginal bone because of the penetration of bacteria into the implant– abutment interface (i.e., compared to an implant without a gap that permits bacterial invasion). It has been claimed that with conical implant–abutment attachment systems, this is not as much of a risk as the gap is much smaller with less leakage at the implant–abutment interface,1,2 thus retarding or preventing bacterial colonization. However, it must be recognized that as of now, there are no endosseous dental implant systems that can provide a complete seal at the implant–abutment interface1,3,4 and so this is still an important clinical issue. Regarding the mechanical properties of implant connections, it has been assumed that different abutment connections might provide greater resistance to displacement that is caused by excessive occlusal forces.5 In this regard it has Correspondence to: C. M. Schmitt; e-mail: [email protected] Contract grant sponsor: Bavarian Association for Scientific Dentistry, Germany (VfwZ) 552 C 2013 WILEY PERIODICALS, INC. V REVIEW ARTICLE been speculated that this displacement will increase stress/ strain on the endosseous implant thus promoting the acceleration of marginal bone loss.6,7 Clearly then, there is need for improvements in the connection systems currently in use but at this time it is unknown as to whether one connection system currently available, might be superior to others. This review was undertaken to determine whether there is any evidence to support the superiority of any connection system over the other. Based on previous claims, it was decided to focus more specifically on the performance of conical (Morse Taper) implant–abutment connection systems and to compare them with each other and to implant systems with nonconical interfaces and to determine whether there might be improved clinical outcomes with one of these systems. MATERIAL AND METHODS This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and MetaAnalyses statement, the recommendations of the Cochrane Handbook for Systematic Reviews and known literature guidelines writing a systematic review.8–11 Several articles related to the in vitro and in vivo performance of conical implant–abutment connection systems were reviewed. The central review questions were as follows: 1. Do implant systems with a conical (Morse Taper) implant–abutment connection compared to nonconical implant–abutment connection systems in vitro show better performances in terms of implant–abutment seal and under mechanical stress/loading? 2. Does the use of a conical (Morse Taper) implant–abutment connection system compared to a nonconical implant–abutment connection system result in less marginal bone level changes and higher implant survival rates? Inclusion and exclusion criteria Studies were included according to the following general inclusion criteria: 1. Publication in an international peer-reviewed journal. 2. Study published in English. 3. Publication not older than 15 years. Additional inclusion criteria for in vitro studies: 1. Only comparative studies with a minimum number of two groups, with one related to the use of a conical (Morse Taper) implant–abutment connection and the other related to the use of another conical or nonconical implant–abutment connection system. 2. Studies investigating implant–abutment seal, particularly microgap formation and bacterial leakage. 3. Studies investigating the performance of the implant– abutment unit under loading conditions in vitro; especially preload/torque loss, load fatigue performance/ JOURNAL OF BIOMEDICAL MATERIALS RESEARCH A | FEB 2014 VOL 102A, ISSUE 2 resistance, bending moment/maximal load resistance and strain/stress distribution in and around the implant– abutment interface with respect to the implant–abutment configuration. Additional inclusion criteria for in vivo studies: 1. Only comparative studies (animal and human trials), with a minimum number of two groups, with one related to the use of a conical (Morse Taper) and the other to a nonconical implant–abutment connection system. 2. In cases of clinical trials only prospective clinical comparative studies with a minimum follow-up of 12 months. 3. Studies investigating marginal bone level changes and implant survival rates. Publications not meeting all mentioned inclusion criteria, using analytical formulas only, or dealing with case reports, case series, abstracts, letters, and narrative reviews were excluded from this systematic review. In the presence of duplicate publications, only the study with the most inclusive data was selected. Search strategy The following electronic databases were searched: 1. The Cochrane Library (up to November, 15th, 2012): a. CDSR (Cochran Database of Systematic Review), b. The Cochrane Central Register of Controlled Trials (CENTRAL), c. The Cochran Review Groups. 2. MEDLINE (up to November, 15th, 2012). 3. EMBASE (up to November, 15th, 2012). Electronic search was carried out using the logical operators: (“dental implant”) AND (“dental abutment”) AND [(“taper”) OR (“cone”) OR (“conical”)]. In order to detect additional trials dealing with conical implant–abutment systems, the databases were searched for major implant systems with a conical implant–abutment connection, including: ITI/ Straumann Dental Implant System (Straumann AG, CH-Basel/ Straumann GmbH, Freiburg, SwitzerR (Dentsply FRIADENT GmbH, Mannheim, land), AnkylosV Germany), BICON Dental Implants (BICON Europe, Sohren, Germany), AstraTech Implant System (AstraTech GmbH, Elz R (Camlog GmbH, Wimsheim, Germany), Germany), ConelogV R (NobelBiocare Ag, Sweden), AlphatecV R BONINobelActiveV R DUOtex (Henry Schein Dental Depot GmbH, tex, AlphatecV Langen, Germany), Neoss Implant System (Neoss GmbH, K€ oln Germany), Neodent Titamax CM, Neodent Alvim II Plus IC (Neodent, Curitiba, Parana, Brazil), and TS Implant System (Osstem Implants GmbH, Eschborn Germany). Therefore the name of the system, respectively, the manufacturer was used as a logical operator and combined with the keywords as follows: (“dental implant”) AND (“dental abutment”) AND (“name of the system” OR “name of the manufacturer”). In addition a hand search was carried out for the last six months in the following journals: Journal of Clinical 553 Periodontology, Journal of Dental Research, Clinical Oral Implants Research, Clinical Implant Dentistry and Related Research, Journal of Periodontology, Journal of Periodontal Research, The International Journal of Oral & Maxillofacial Implants, Journal of Cranio and Maxillofacial Surgery, Implant Dentistry, International Journal of Prosthodontics, Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology, The International journal of Periodontics & Restorative Dentistry, The Journal of the American Dental Association, The Journal of Prosthetic Dentistry, American Journal of Dentistry, Journal of Esthetic and Restorative Dentistry, Quintessence International, Periodontology 2000, British Journal of Oral and Maxillofacial Surgery, Journal of Oral and Maxillofacial Surgery, Journal of Cranio Maxillofacial Surgery, Journal of Canadian Dental Association, and Journal of Oral Implantology. The search and screening process was carried out by two independent examiners (CS, GN) to minimize the potential for reviewer biases. After electronic search all titles, key words and abstracts were screened. Irrelevant studies or studies not meeting the inclusion criteria were excluded. All full texts of the remaining articles were acquired for the second screening. The references of all selected publications were additionally checked for further relevant data. In cases of missing or insufficient data the corresponding authors were contacted via e-mail. After detailed full text examination and agreement between examiners further articles were excluded. All remaining studies were included in this systematic review. The references were managed with specific bibliR , NY). ographic software (EndNoteX4, ThomsonReutersV Data extraction Data extractions were performed independently by the two reviewers (CS, GN) using data extraction tables. In cases of disagreements, the data were double checked with the original data. The following data were extracted from the selected articles: (1) authors, (2) title, (3) year of publication, (4) journal name, (5) implant–abutment connection, (6) implant system, (7) number of implants per group, (8) study design, (9) primary objective, (10) secondary objectives, (11) methods, and (12) results. RESULTS The initial electronic literature search identified 468 publications (Fig. 1). Hand search did not provide any additional studies. Review of all titles, key words, and abstracts led to the exclusion of 345 studies, which left 123 studies for full text screening. After full text evaluation, 52 articles were excluded, as they did not fulfill inclusion criteria. Reference screening revealed eight additional studies. A total of 79 studies were analyzed in detail for potential inclusion in the review. Twenty-seven additional studies were excluded for the following reasons: eight studies did not include a comparable control group with another conical or nonconical implant–abutment connection system,12–19 two studies used implant and abutment replicas as a control group,20,21 three studies used analytical formulas only,22–24 three studies did 554 SCHMITT ET AL. FIGURE 1. Study selection process. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.] not investigate a conical implant–abutment connection, five studies did not meet primary objectives,25–29 one study had a follow-up of only 6 months,30 one study had a retrospective design,31 and four in vivo studies used implants with machined surfaces.32–35 The remaining 52 studies were included in the review. Thirteen out of 52 studies dealt with implant–abutment seal performance, particularly focusing on bacterial colonization, saliva leakage, endotoxin ingress as well as overall microleakage based on dye penetration. Thirty studies concentrated on stress and loading performance/resistance of the implant–abutment unit. Nine studies were carried out in vivo, and there were five animal and four clinical (i.e., human) trials. Regarding the secondary outcome of some in vitro studies, there was overlap between groups.2,3,5,36,37 If applicable and meeting the inclusion criteria, a double assessment was conducted concerning the secondary outcome.2,3,5,36,37 The study characteristics and outcomes for in vitro studies are detailed in Tables I and II, and for in vivo studies in Tables III and IV. In vitro Seal performance. Eight trials studied the bacterial leakage of the implant–abutment interface.1,3,4,36,38–41 Following PERFORMANCE OF CONICAL IMPLANT–ABUTMENT CONNECTION SYSTEMS System # Samples Pr. Objective 10 per group, 5 According to the recommendaper bacterial tion of the species manufacturer P. aeruginosa, A. actinomycetemcomitans N.a. S. aureus P. aeruginosa (PS), A. actinomycetemcomitans (AA) Human saliva Used Bacteria/ Dyes N.a. N.a. Sec. Objectives 20 per system Titamax II Plus 20 Bacterial leakage Bacterial leakage Teixeira et al., Internal cone, Titamax CM, Ncm, Titamax into the implant- from the 2011 internal hex Titamax II Plus (10 per CM 32 Ncm, abutment implant-abutexperiment) (Neodent) (recommended interface ment interface by the manufacturer) Bacterial leakage from the implant-abutment interface Bacterial leakage from the implant–abutment interface According to the Saliva leakage recommendainto the implant– tion of the man- abutment ufacturer (20 interface under Ncm) loaded and unloaded conditions Insertion torque Ankylos (Dents- 10 per system According to the ply Friadent), recommendaReplace Select tion of the (Nobel Biomanufacturer care), Bone System Internal cone, Universal II HI internal hex and CM, (Implacil De Bortoli) Morse taper, SIN, Sistema de 20 per group, internal and Implante 10 loaded external hex Nacional and 10 unloaded Connection Assenza et al., Internal cone, 2011 internal trilobed, cemented Tripodi et al., 2012 Nascimento et al., 2012 Author/ Year SEAL PERFORMANCE TABLE I. Seal Performance Result Implant abutment Contamination: connection and External hex: incubation in Loaded 10 out human saliva. of 10, unloaded Detecting saliva 3 out of 10 Interleakage. Half of nal hex: Loaded the specimens: 10 out of 10, Cycling with 120 unloaded 4 out N, 500,000 of 10, Morse cycles at 1.8 Hz taper: Loaded 9 out of 10, unloaded 1 out of 10 Bacterial inocula- For PS inoculation of the tion: 2 out of 5 implant and in the conical abutment congroup and 2 out nection and of 5 in the interdetecting bactenal hex group, rial leakage for AA: 0 out of five in the conical and 3 out of 5 in the internal hex group Bacterial inocula- Bacterial leakage: internal conical tion of the 1 out of 10, inimplant and ternal trilobed 6 abutment conout of 10, nection, and cemented 0 out measuring bacof 10 terial leakage 1. Bacterial con- Into: Conical 70% tamination and internal hex before and 2. af- 100% leakage. ter implant-abut- From: Conical ment connec77.7% and intertion, incubation nal hex 100% and colony leakage growth calculation Method System Aloise et al., 2010 # Samples Insertion torque Pr. Objective Sec. Objectives Used Bacteria/ Dyes Internal cone, Bicon Implant 10 per system Ankylos 25 Ncm, Bacterial leakage N.a. internal cone System Bicon tapped, from the (Bicon), Anky(recommended implant-abutlos (Dentsply by the ment interface. Friadent) manufacturer) S. sanguinis 11 per system Morse Taper 1: 35 Bacterial leakage Preload loss after S. sanguinis (6 with and 5 Ncm, Morse into the implant thermal cycling without Taper 2 and abutment interand mechanical loading) external hexago- face subjected fatigue nal: 15 Ncm, to thermal cy(recommended cling and meby the chanical fatigue. manufacturer) Internal cone, Ankylos (Dents- 14 per system Ankylos 25 Ncm, Bacterial leakage Torque value loss E. coli four groove ply Friadent), Bone level 35 into the implant- after loading internal cone Bone level (ITI Ncm, (recomabutment interStraumann) mended by the face during manufacturer) loading Connection Ricomini Filho Internal cone 1 Not mentioned et al., 2010 (one piece), internal cone 2 (two pieces), external hex, locking taper Koutouzis et al., 2011 Author/ Year SEAL PERFORMANCE TABLE I. Continued Result Implant abutment Ankylos: 1 out of connection, 14, mean CFUs loading in E. 14.07652.56, coli medium, torque increase disconnection (2.8563.23 measuring loos- Ncm), Bone ening torque, level (ITI): 12 incubation and out of 14, mean measuring CFUs CFUs 184.646242.32, torque decrease (-5.0062.77 Ncm) Connection, ther- Bacterial leakage mal cycling and after loading: mechanical faMorse Taper 1 tigue testing, (67%), Morse sterilization and Taper 2 (50%), contamination external hexagoto bacterial menal (0%), locking dium, detorque taper (60%) Premeasurements load loss after and SEM cycling: Morse analysis Taper 1 (12.5%), Morse Taper 2 (-23.3%), external hexagonal (-23.1%). Inoculation S. san- Bactarial leakage: Ankylos 20%, guinis, connectBicon 20%. ing abutment and implant, incubation and proof of bacterial presence or absence Method Internal cone Ankylos (Dents(2x), external ply Friadent), OsseoSpeed flat, internal (Astratech), flat Standard ITI (ITI Straumann, Nobel Replace Tapered Groovy (Nobel Biocare) Internal cone, Ankylos and manipulated manipulated internal Ankylos cone, tri(Dentsply Friachannel indent), Nobel ternal Replace Select connection (Nobel Biocare) Baixe et al., 2010 Tesmer et al., 2009 System Internal cone, OsseoSpeed internal cone (AstraTech), Ankylos (Dentsply Friadent) Connection Harder et al., 2010 Author/ Year SEAL PERFORMANCE TABLE I. Continued According to the recommendation of the manufacturer Insertion torque Molecular leakage N.a. of endotoxin along the implant abutment interface Pr. Objective Sec. Objectives Used Bacteria/ Dyes Method Result LPS of Salmonella enterica Inoculation of Endotoxin detecimplant with tion in both LPS, connection groups after 5 to abutment and minutes. Signifiincubation, encant less endodotoxin detectoxin concentration and tion (mean) for measuring conOsseoSpeed centration over units over the time (168h) whole examination period Longitudinal cut- The mean micro5 per system Nobel 35 Ncm, ITI Microgap between Microgap compar- N.a. gap was larger ting and scaning titanium and implant and 15 Ncm, Astra for flat-to-flat ning electron zirconia abutment 25 Ncm, Ankyinterface sysmicroscopy abutments los 15 Ncm (rectems compared ommended by to conical interthe face systems, manufacturer) zirconia abutments showed smaller microgaps than titanium abutments 10 per system Ankylos and Bacterial invasion N.a. A. actinomycetemcomitas, Implant abutment Bacterial contamimanipulated into the implant P. gingivalis connection, con- nation Ankylos: Ankylos 25 abutment tamination with (Aa 3/10, Pg 0/ Ncm, Nobel interface bacterial solu10, median Replace Select tion (Aa and CFUs; Aa 0, Pg 35 Ncm, (recomPg), disconnec0), Nobel mended by the tion, incubation Replace select: manufacturer) and detecting (Aa 9/10, Pg 9/ bacterial 10, CFUs; Aa contamination 24.5, Pg 12), manipulated Ankylos: (Aa 10/ 10, Pg 10/10, CFUs; Aa 81, Pg 55) 8 per system # Samples Insertion torque Gross et al., 1999 Internal cone OsseoSpeed 10 per system According to the (3x), div. (Astratech), recommendaexternal flat Ankylos, Friation of the manufacturer (7x), flat lit-2, IMZ 1internal (Dentsply Friacone, flat 1 dent), Bonefit internal siliconical and con washer synOcta (ITI Straumann), Branemark (Nobel Biocare), Semados (Bego Semados), HaTi (Ledermann), Calcitek Implants # Samples Jansen et al., 1997 System Internal cone, Standard SLA 5 per system According to the trilobed inter- implant (ITI, recommendanal, internal Straumann), tion of the hex Replace Select manufacturer (Nobel Biocare), Intralock short collar implant (Intra-lock Int.) Internal cone, ITI (Straumann), 3 per system (1 10 Ncm, 20 Ncm external hex 3i, CeraOne per torque and according (2x), spline and Steri-Oss group) to the recomconnection (Nobel Biomendation of care), Spline the (Sulzer manufacturer Calcitek), Connection Coelho et al., 2008 Author/ Year SEAL PERFORMANCE TABLE I. Continued N.a. Dye leakage over time Used Bacteria/ Dyes Gentian violet dye Toluidin Blue dye Bacterial seal from Microgap between E. coli implant- abutimplant and ment interface abutment N.a. Sec. Objectives Sealing capability of implant system Pr. Objective Result Contamination of Total release after implant inter144h: ITI 55%, face, connection Intra-lock 22% to abutment and and Replace measuring dye Select 100%. leakage over time with spectrophotometric analysis Contamination of Leakage increased implant interin all systems face, connection over time with to abutment and no significant measuring dye differences after leakage over 80 minutes, time with specleakage trophotometric decreased siganalysis nificantly as tightening torque increased to recommended values Bacterial inocula- All systems tion of the inner showed bactepart of the rial leakage of implant, abutthe implant ment connecabutment intertion, cultivation face after 5 and detection of days, the micro bacterial leakage gap was less over time (14 than 10 mm in days), microgap all systems, condetection with ical connection SEM systems showed the smallest micro gap Method Connection System Conexao Implant Systems (Conexao Sistemas de Protese) Branemark CeraOne (Nobel Biocare), 3i Osseotite-STA (3i, Biomet), Replace SelectEasy (Nobel Biocare), Lifecore Starge1-COC (LC Lifecore Biomedical) Internal cone, internal hex, external hex External hex, external hex, cam tube, Internal cone Ribeiro et al., 2011 Quek et al., 2008 Load fatigue performance/ resistance Seetoh et al., Internal cone, Ankylos (Dentsply 2011 internal hex1 Friadent), Lifecone, internal core PrimaConfour groove1 nex (Keystone cone Dental), Bone Level (ITI Straumann) Author/ Year STRESS/LOADING PERFORMANCE TABLE II. Stress/Loading Performance 15 per system, (5 per group: recommended torque and -20% and 120% Ncm) 30 per system 10 per system, 5 per group (titanium (Ti) and zirconia (Zr) abutments) # Samples Branemark 28 Ncm, 3i 32 Ncm, Replace Select 35 Ncm, Lifecore 30 Ncm (recommended by the manufacturer and -20%/ 120%) 30 Ncm According to the recommendation of the manufacturer Insertion torque Sec. Objectives Load fatigue perform- Effect of decreasance/ resistance of ing/ increasing different implanttightening torabutment connecque values tion systems and about 20% region and mode of failure Load fatigue perform- Determine the failance/ resistance of ure mode and three implant- abutregion ment connection systems Load fatigue perform- Fatigue performance/ resistance of ance of Ti and different implantZr abutments abutment connecand determine tion systems failure mode and region Pr. Objective Fatigue loading until failure or maximal cycles (5 3 106 cycles). Examination of the fracture region and surface with SEM Fatigue loading and calculating of the F50 value (at which 50% of the samples failed and 50% ran out), stereomicroscopy and SEM analysis of fracture region Fatigue loading until failure of the implant abutment specimens or maximal cycles (10 Hz, 5 3 106 cycles). SEM analysis of fracture region Method No significant difference between the Ti abutments tested for the three systems. Straumann Zr abutments showed significant better load fatigue resistance than Ankylos and PrimaConnex implantsabutment systems. External hexagonal: F50, 53.567.8 N, internal conical: F50, 4462.49 N, internal hexagonal: F50, 4563.40 N. In 24 out of 30 cases fracture region was observed in the threaded part of the abutment. No statistical significant differences in the number of cycles to failure between the four systems when recommended torque values were used; failure location is system specific and always occurs at the weakest point of the implant abutment connection. Result System ITI Solid and SynOcta implants (ITI Straumann) Branemark (Nobel Biocare), ITI Solid screw (ITI Straumann) ITI Standard (S) and synOcta (O) implants (ITI Straumann) 1 Solid (S) and synOcta (O) abutments (ITI Straumann) Connection Internal cone, internal octagon Internal cone, external hex Internal cone, internal octagon Author/ Year Cehreli et al., 2004 Khraisat et al., 2002 Perriard et al., 2002 STRESS/LOADING PERFORMANCE TABLE II. Continued 20 specimens for O-O, 10 for O-S and 10 for S-S combination (AbutmentImplant) 7 8 # Samples Sec. Objectives Load fatigue perform- Mode and region ance/ resistance of of failure two different implant- abutment connection systems Load fatigue perform- Fracture mode and region, ance/ resistance of peak stresses in different ITI the implantimplant-abutment abutment connections connection 40 Ncm Load fatigue perform- Tightening torque ance/resistance of loss after different implantloading abutment connection systems Pr. Objective Branemark 32 Ncm, ITI 35 Ncm (recommended by the manufacturer) 35 Ncm (recommended by the manufacturer) Insertion torque Result Fatigue loading Solid abutments (500,000 cycles, showed significant Periotest value higher RTVs than (PTVs) measuresynOcta abutments, ments after evboth implant abutery 100,000 ment connections cycles), after tershowed comparamination reble high fatigue moval torque resistances value (RTV) measurement Fatigue loading ITI solid screw: no until failure of failures, Branethe implant mark: fracture abutment specibetween 1,778,023 mens or maxiand 1,733,526 mal cycles cycles (significant (1,800,000 difference); fraccycles), fracture tures occurred surface analysis between the with SEM threaded and unthreaded parts of the abutments Implant abutment S-O connection more connection and resistant to force fatigue loading application; S-O (step procedure, combination supeand calculating rior to O-O and SF50), FEM detectS; S-S- and O-O comparable; in ing stress peaks cases of fracture no in implant-abutpreferential locament tion detectable in connection all three groups; stresses in the implant-abutment interface: O-O more stresses than S-O and S-S Method Connection System OsseSpeed (Astratech), Branemark (Nobel Biocare) Internal cone, external hex Norton et al., 1997 Tightening/ loosening torque, cold welding Osstem Implant Park et al., Internal cone, inSystems (US II, 2010 ternal cone1 SS II, GS II) external colar, external hex OsseoSpeed (1-piece Uni-abutment St and 2piece Profileabutment ST) (Astratech) Internal cone, internal hex1 cone Norton et al., 2000 Bending moment/ maximal load resistance Coppede et al., Internal cone, inAlvim II Plus 2009 ternal hex implants with internal hex (IH) and with internal cone (IC) (Neodent Implants) Norton et al., Internal cone, inOsseoSpeed 2000 ternal cone (Astratech), standard ITI (ITI Straumann) Author/ Year STRESS/LOADING PERFORMANCE TABLE II. Continued 10 per system, 5 per group (titanium and tungsten carbide carbon coated titanium abutments) 6 6 30 Ncm Compression force tightening abutment to implant and screw removal torque before and after cycling Astra 8 Ncm, Brane- Resistance to bendmark 20 Ncm ing moment/ maxi(recommended by mal fatigue the manufacturer) resistance 1-piece abutment 15 Resistance to bendNcm, 2-piece ing moment/ maxiabutment 25 Ncm mal fatigue (recommended by resistance the manufacturer) Astra 25 Ncm, ITI 35 Resistance to bendNcm (recoming moment/ maximended by the mal fatigue manufacturer) resistance 6 Pr. Objective IH implants 10 Ncm, Resistance to bendIC implants 20 ing moment/ maxiNcm (recommal fatigue mended by the resistance manufacturer) Insertion torque 10 # Samples N.a. N.a. N.a. N.a. N.a. Sec. Objectives Astra: Mean Pb 4176 Nmm, mean Mb 5507 Nmm, significant higher bending moments at plastic deformation and failure than ITI: Mean Pb 2526 Nmm, mean Mb 3269 Nmm Astra (1-piece): Mean Pb 4176 Nmm, mean Mb 5507 Nmm; Astra (2-piece): Mean Pb 4049 Nmm, mean Mb 6281 Nmm, no statistical significant differences Astra: Mean Pb 1315 Nmm, mean Mb 2030 Nmm; Branemark: Mean Pb mean 645 Nmm , mean Mb 1262 Nmm , significant difference between systems IC: 90.58 6 6.72 kgf (MDF), no fracture, IH: 83.876 4.94 kgf (MDF), 79.8664.77 kgf (FF), significant difference for MDF Result Measuring comAll systems showed pression force preload loss after and tightening initial tightening. and removal torExternal hexagonal que before and connection showed after loading significantly higher (106 cycles). preload loss after loading than the two conical connections 3 point bending test until failure or maximum load, measuring plastic bending moment (Pb) and maximal bending moment (Mb) 3 point bending test until failure or maximum load, measuring plastic bending moment (Pb) and maximal bending moment (Mb) Maximal loading until failure, measuring maximal deformation force (MDF) and fracture force (FF) 3 point bending test until failure or maximum load, measuring plastic bending moment (Pb) and maximal bending moment (Mb) Method System Alvim CM implants and Universal abutment CM oneand two-piece (Neodent) OsseoSpeed (Astratech), BioLok (Bio-Lok), Branemark (Nobel Biocare), Screw-vent (Zimmer Dental) Standard and synOcta ITI (ITI Straumann) Connection Internal cone, internal cone Internal cone, external hex (2x), internal hex1 cone Internal cone, internal octagon Author/ Year Richiardi Copedde et al., 2009 Piermatti et al., 2006 Ding et al., 2003 STRESS/LOADING PERFORMANCE TABLE II. Continued 12 ITI standard, 24 synOcta (12 with solid and 12 with synOcta abutment) 10 34 per implantabutment system, 17 per group (loading and no loading) # Samples 35 Ncm (recommended by the manufacturer) 32 Ncm 20 Ncm solid abutment, 10 Ncm two-piece abutment (recommended by the manufacturer) Insertion torque Repeated torque/ reverse torque values of each system and implant-abutment combination Removal torque in combination with loading Effect of loading on the abutment removal torque Pr. Objective Method Result Effect of repeated Measuring reLoading increased reinsertion/ removal torque afmoval torque; two moval cycles on ter repeated piece system had the abutment reinsertion/ reto be removed in moval torque moval and after two steps with torloading (1,325 que gain of the seccycles), SEM ond piece after loading (cold welding); increasing number of abutment insertion/removal decreased removal torque values N.a. Off axis loading of Astra showed significant higher torque the specimens loss than other sysand recording tems under loading removal torque conditions, screw every 250.000 design seems so cycles up to 106 cycles be an important factor influencing the loosing torque Maximal failure Measuring Initial removal torque load repeated in/ out of solid abutments torque values combined with and maximal standard and synbending Octa implants were moment, SEM significantly higher than the initial torque removal of the synOcta implant1 abutment, solid abutments with both implant types showed significant higher load resistance Sec. Objectives Saidin et al., 2012 Internal cone, trilobe, internal hex, internal octagon N.a. (simulation) Stress/ strain distribution Yamanishi External hex, inter- N.a. (simulation) et al., 2012 nal cone, internal straight Astratech (diame- 5 for Astra, 4 for ters 3.5 and 5.0), ITI standard ITI (ITI Straumann) Internal cone, internal cone, internal cone Norton et al., 1999 N.a. N.a. 3 Weiss et al., 2000 # Samples System Standard ITI (ITI Straumann), SpectraClone (Alpha Bio), Spline, Integral and Omnitek (Calcitek), SteriOss, Branemark (Nobel Biocare) Connection Internal cone (2x), external hex (3x), internal octagon, flat rim Author/ Year STRESS/LOADING PERFORMANCE TABLE II. Continued Insertion torque Pr. Objective Comparison of torque loss as a result of multiple consecutive closures within and between the systems N.a. N.a. Effect of implantabutment connection on micromotion and abutment stress distribution Effect of implant abutment design on abutment micromovement, implant- abutment interface and periimplant stress distribution Group 1: low torque Torque loss after dif(4-50 Ncm), ferent tightening Group 2: high tortorques in wet and que (100- 300 dry environment Ncm) for different implant-abutment connections According to the recommendation of the manufacturer Method Result Fenite element analysis method (FEM), simulating an oblique load Fenite element analysis method (FEM), simulating axial and oblique loads N.a. External hex connection: Largest amount of abutment movement, higher labial bone stresses; Internal conical: Lowest abutment movement and low labial peri-coronal bone stresses Stress concentrates at vertices of nonconical abutments; conical abutments showed more uniformly distributed stresses; internal hex connection showed the greatest stresses, followed by internal conical, octagonal and the trilobed connection. 200 repeated con- Significant higher secutive closing/ maintaining torque opening cycles values in either and measuring conical frictional the torque elements or intervalues locking lines, removal torque declined for all systems progressively up to 200 c/o cycles Measuring differAll combination ent tightening showed comparaand the resultble removal toring removal torques in wet and ques in wet and dry environments; dry cold welding did environments not occur between 20 and 40 Ncm; surface area of interface seems to influence torque loss N.a. N.a. N.a. Sec. Objectives 3 N.a. Conexao Implant System (Conexao Systemas de Protese) Neodent Implant System Internal cone, internal hex, external hex Internal cone, internal hex, external hex Nishioka et al., 2011 Pessoa et al., 2010 # Samples 5 System Internal hex, exter- Conexao Implant nal hex, internal System (Conoctagon 1cone, exao Systemas internal cone, de Protese), ITI internal locking (Straumann), taper Bicon Connection Pellizzer et al., 2011 Author/ Year STRESS/LOADING PERFORMANCE TABLE II. Continued N.a. According to the recommendation of the manufacturer According to the recommendation of the manufacturer Insertion torque N.a. Sec. Objectives Stress/ strain in periimplant bone and influence on abutment and implant stability (before and after osseointegration) Influence of connection type on bone-to-implant relative displacement and abutment microgap Strain/ stress distribu- Effect of implanttion around abutment conimplants nection and implant fixture alignment Strain/ stress distribution around implants Pr. Objective Result Photoelastic analy- Axial load: Greatest sis under vertistress concentracal and oblique tion in the cervical loading and apical thirds. Oblique load: At the implant apex and in the cervical adjacent to the load direction. Internal octagon1 cone presented the lowest stress concentrations, external hex exhibited the greatest stresses. Strain gauge Statistically signifianalysis cant difference comparing the implant- abutment connections, Morse Taper and internal hexagon did not reduce strain around implants, no statistical significance in the placement configuration Conical connection Fenite element showed a signifianalysis method cant higher abut(FEM), simulatment stability, the ing non-axial smallest microgap loading for immediate loaded and the lowest and osseointestress in the abutgrated implants ment screw; marginal bone stresses were comparable for the simulation of immediate placed implants and lower for Morse Taper connection implants after osseointegration Method Neodent Implant System Frialit-2, Ankylos (Dentsply Friadent) synOcta, Monoblock ITI (ITI Straumann), Bicon Implants (Bicon), OsseoSpeed (AstraTech) Frialit-2 (Dentsply Friadent), Bicon, standard ITI Straumann Internal cone, internal hex, external hex, one-piece implant Internal cone, internal hex Internal cone, internal cone, internal cone, one-piece implant Internal cone, internal hex, internal cone Bernardes et al., 2009 Quaresma et al., 2008 Akca et al., 2008 Lin et al., 2007 System Connection Author/ Year STRESS/LOADING PERFORMANCE TABLE II. Continued N.a. 2 per system N.a. 4 # Samples N.a. Not mentioned N.a. Not mentioned Insertion torque Force transmission in the peri-implant bone region of implants with different conical implant-abutment connections Strain/ stress distribution around implants influenced by implant-abutment connection Strain/ stress distribution in the prosthesis, abutment, implant and surrounding alveolar bone under different loading conditions Peri-implant stress fields generated from four different implant-abutment interfaces Pr. Objective N.a. N.a. N.a. N.a. Sec. Objectives Result No significant difference under centered axial loading, smallest periimplant stress field for internal hexagonal connection under off-center loads; Internaltaper interfaces presented intermediate results Fenite element Conical abutment analysis method showed lower (FEM), simulatstresses on alveolar ing different verbone and prothesis tical occlusal and higher stresses forces on abutment. Internal hexagonal abutment showed higher bone stresses and lower abutment stresses The internal conePhotoelastic and implants showed strain-gauge similar interface analysis under force transfer charvertical and acteristics that oblique forces resemble a one-piece implant system Fenite element Internal conical conanalysis method nection performed better as a force(FEM), simulattransmission meching different ocanism than other clusal loads systems, conical systems showed lower interface and marginal bone stresses than internal hexagonal connection system Photoelastic strain analysis under different vertical center and offcenter loading conditions Method Branemark (Nobel Biocare), ITI solid, synOcta (ITI Straumann) ITI and hypothetical butt joint ITI (ITI Straumann) Internal cone, external hex, internal octagon Internal cone, external hex Internal cone, external flat top Alkan et al., 2004 Merz et al., 2000 Hansson et al., 2000 Kitagawa et al., Internal cone, 2005 external hex ITI Straumann, Astratech, Branemark (Nobel Biocare) Internal cone, internal cone, external hex Cehreli et al., 2004 Ankylos (Dentsply Friadent), Branemark (Nobel Biocare) N.a. (simulation) System Connection Author/ Year STRESS/LOADING PERFORMANCE TABLE II. Continued N.a. N.a. N.a. N.a. 2 per system # Samples Ankylos 20 Ncm, Branemark 32 Ncm (recommended by the manufacturer) N.a. Simulated with torque of 35 Ncm according to the recommendation of the manufacturer Simulated according to the manufacturers recommendations Not mentioned Insertion torque Dynamic behavior (screw loosening) of different implant-abutment connections Stress distribution around implants with conical and external flat implant abutment connections Mechanics of two different implantabutment connections Stress distribution of preloaded dental implant screws in different implantabutment joint systems under simulated occlusal forces Force transfer characteristics of different implant abutment connections Pr. Objective N.a. N.a. N.a. N.a. N.a. Sec. Objectives Result Fenite element analysis method (FEM) comparing the movement of the taper-and external type-joint model The external typejoint model showed rotation movement, the taper type-joint showed no movement Strains around Branemark implants were lower than around Astra and ITI implants particularly under vertical loads 3-dimensional fen- In all systems maxiite element analmum stress was ysis method examined between (FEM), 3 simuthe shank and first lating occlusal thread of the abutloads (horizonment; stress tal, vertical, increased in all sysoblique) tems under loading conditions Fenite elment Significant higher analysis method stress in the butt (FEM), simulatjoint connection ing vertical and tightening the abutdifferent off-axis ment to the loads implant, taper connection compensated high forces, butt joint showed more stress in the implant abutment connection Significant decrease Fenite element in the peak boneanalysis method implant interfacial (FEM), simushear stress in conlated axial ical implant abutloading ment connections, external flat top showed high marginal peri-implant stress peaks, conical system showed lower marginal stress peaks Photoelastic and strain gauge analysis with vertical and oblique load application Method Astratech, Branemark (Nobel Biocare) Astratech, Branemark (Nobel Biocare) Astratech, Branemark (Nobel Biocare) Internal cone, external hex Berglundh et al., 2005 Abrahamsson Internal cone, et al., 1998 external hex Abrahamsson Internal cone, et al., 1996 external hex Beagle dog Beagel dos Beagle dog Ankylos (Dentsply Mongrel Friadent), Branedog mark (Nobel Biocare) Internal cone, external hex Weng et al., 2011b Animal model Ankylos (Dentsply Mongrel Friadent), Branedog mark (Nobel Biocare) System Internal cone, external hex Connection Weng et al., 2011a Author/Year ANIMAL STUDIES TABLE III. Animal Studies 5 5 6 8 6 # Animals Healing 10 implants per system 9 implants per system 24 implants per system submerged submerged submerged 4 groups a 8 submerged implants (conical equicrestal and subcrestal, external hexagonal crestal and subcrestal) 4 groups a 6 nonsubmerged implants (conical equicrestal and subcrestal, external hexagonal crestal and subcrestal) # Implants No No Yes No No Loading Histological observation N.a. N.a. Secondary objective Histological obser- Soft tissue vation periresponse implant tissue, around marginal bone implants to loss plaque formation Histological obser- Soft tissue vation periaround implant tissue, implants marginal bone loss Radiographical marginal bone loss Radiographical marginal bone loss Radiographical marginal bone loss Primary objective Marginal bone loss: Astratech 0.5760.44 mm, Branemark 0.6260.12 mm Marginal bone loss Conical: equicrestal (0.6860.59 mm), subcrestal (0.7660.49 mm) External: equicrestal (1.3260.49 mm), subcrestal (1.8860.81mm) Marginal bone loss Conical: equicrestal (0.4860.66 mm), subcrestal (0.7960.93 mm) External: equicrestal (0.6960.43mm), subcrestal (1.5660.53 mm) Marginal bone loss: Astratech 0.0960.16 mm, Branemark 0.7760.42 mm Marginal bone loss: Astratech 0.6460.44 mm, Branemark 0.6460.72 mm Results 568 SCHMITT ET AL. PERFORMANCE OF CONICAL IMPLANT–ABUTMENT CONNECTION SYSTEMS Connection 45 177 24 month 12 month Ankylos (Dents- CT ply Friadent), Seven Sweden and Martina Implants Nobel Active RCT (NA) internal multicenter and study external, Nobel Replace (NR, Nobel Biocare) Internal cone, external hex Internal cone, external cone external trilobe Crespi et al., 2009 Kielbassa et al., 2009 26 40 # Patients 24 month 12 month Follow-up Astratech, Bra- CT nemark (Nobel Biocare), ITI (Straumann) RCT Study Design Internal cone, external hex Samo Smiler Implants, Biospark System Bilhan et al., 2010 Pieri et al., Internal 2011 cone, internal hex Author/ Year HUMAN STUDIES TABLE IV. Human Studies Placed Healing Loading Objective Results immediately nonsubmerged immediately Clinical and Marginal bone radioloss: Conical graphical 0.260.17 outcome mm, internal (marginal hex bone 0.5160.24 loss), mm Implant implant success: Consuccess ical 94.7%, internal hex 100% 42 (Astra), 36 delayed submerged delayed Soft tissue, Marginal bone (Branemark), marginal loss: Astra29 (ITI) bone loss, tech 0.6660.1 implant mm, ITI survival 0.860.1 mm, Branemark 1.160.1 mm, Implant survival: all 100% 34 (Branemark), Immediately nonsubmerged Immediately Marginal Marginal bone 30 (Ankylos) bone loss, loss: Conical implant 0.7360.52 survival mm, external hexagonal 0.7860.45 mm Implant survival: both 100% 117 (internal delayed nonsubmerged immediately Marginal Implant surNA), 82 bone loss vival: Internal (external and soft NA 96.6%, NA), 126 tissue external NA (NR) behavior, 96.3%, NR implant 97.6% Marsurvival ginal bone rate loss: Internal NA 0.9561.37 mm, external NA 0.6460.97 mm, NR 0.6361.18 mm 40 (20 per group) # Implants REVIEW ARTICLE bacterial species were used: Escherichia coli, Aggregatibacter actinomycetemcomitans (Aa), Porphyrmonas gingivalis (Pg), Streptococcus sanguinis (Ss), Pseudemonas aeruginosa (Pa), and Streptococcus aureus (Sa). One study examined microbacterial endotoxin leakage from the implant–abutment interface using lipopolysaccharides (LPS) from Salmonella enterica (Se).42 Only Nascimento et al. examined human saliva leakage of the implant–abutment interface.43 Outcome of included studies showed that 100% seal of the implant– abutment interface to the outside could not be achieved regardless of the implant–abutment connection used.4,38,39 Even if an abutment was tightened to an implant under sterile conditions, bacterial invasion into the interface was still demonstrated in most cases (Table I).1,40 Only one study demonstrated 100% bacterial seal using the Ankylos implant–abutment unit to ingress/colonization with Pg. However, when another organism, Aa, was tested it was demonstrated that this particular bacterial species could still penetrate into the AnkylosV implant–abutment interface.1 Bacterial leakage was also shown in all implant– abutment connection systems under loading in vitro.36 In contrast, Ricomini Filho et al. reported no leakage at all when testing the external hex implant–abutment connection system using Ss.3 Two studies evaluated the implant–abutment seal using dyes (toluidine blue and gentian violet) by measuring particle absorption with spectrophotometric analysis.28,44 Coelho et al. documented significantly lower dye leakage with the Morse Taper and internal hexagonal connection as compared to the tri-lobed internal connection system. Leakage was recorded in all systems and decreased significantly as tightening torque was increased to the values recommended by the manufacturers.28 In summary: the performance of the seal was (1) different in every implant system in vitro regardless of the connection, and (2) there appeared to be significantly less bacterial contamination in implant systems using a pure conical implant–abutment connection as compared to other connection systems.1,4,36,40,41 However, although conical implant–abutment connection systems were able to reduce bacterial contamination significantly, they were still unable to prevent leakage of microbial endotoxin into the seal/gap area.42 Nascimnento et al. showed siginficant less human saliva penetration to the implant–abutment interface in conical conncetion systems.43 Microgaps were detected in all systems with the scanning electron microscope (SEM), but were generally less than 10 mm for all connections tested in this manner.39 The mean microgap was significantly larger for flat-to-flat interface systems compared to conical interface systems.45 Using finite element analysis (FEM),46 Merz et al. documented the formation of a microgap for external hexagonal connection systems on the tension side of the implant under oblique or horizontal loading simulation. Pessoa et al. also demonstrated microgap formation on tension sides for internal hexagonal and external hexagonal connection systems. Conical implant–abutment systems did not appear to develop microgaps.2,37 R JOURNAL OF BIOMEDICAL MATERIALS RESEARCH A | FEB 2014 VOL 102A, ISSUE 2 Stress/load performance. All studies dealing with stress or loading performance of the implant–abutment unit were included in this part of the review. Depending on the primary outcome, the studies were summarized under the following subtopics: (1) load/fatigue performance, respectively, and resistance. There were six studies in this category,47–52 (2) bending moment/ maximal load resistance (4 studies53– 56 , (3) preload loss (tightening/ loosening torque) and cold welding, consisting of six studies,57–62 and (4) stress and strain distribution in and around the implant–abutment interface, consisting of 14 studies.2,5–7,37,63–71 Regarding the secondary objectives, there was an overlapping between the four subtopics in few studies. The studies were assessed for both objectives if applicable and meeting the inclusion criteria (Table II). Load fatigue performance/ resistance. In the investigation of load fatigue performance, specifically resistance of the different implant–abutment connection systems, five trials studied the mode of abutment failure using stereomicroscopy or SEM.47–52 Although primary outcomes were different in the studies, they all focused on the issue of load fatigue performance/resistance. In relation to this Seetoh et al., Quek et al., and Khraisat et al. measured the fatigue loading of the specimens until failure or to a maximum number of cycles (5 3 106, 5 3 106 and 1.8 3 106 cycles).47,49,51 Cehreli et al. tested implant–abutment performance using a maxiR mum number of 500,000 cycles and used the PeriotestV R Values (PTVs) system to measure the so-called PeriotestV over 100,000 cycles of loading/unloading. Ribeiro et al. and Perriard et al. calculated the F50 value at which 50% of the samples failed and 50% run out.48,50,52 Cehreli et al. were unable to demonstrate failure of either the Morse Taper or the internal octagonal attachments used for implant–abutment connections, even after 500,000 loading/unloading cycles. There was a trend towards increased values for PTV for both implant–abutment connection designs and there was no significant difference between either connection systems in this regard.50 After the application of 1.8 3 106 to 5 3 106 cycles, several failures occurred.47,49,51 Khraisat et al. reported a significant difference between the Morse Taper (ITI Straumann) and external hexagonal connection system (Branemark), in that no fractures were noted for the Morse Taper group while the mean fracture rate for the external hexagonal groups was somewhere between 1733 and 1778 cycles.51 After 5 3 106 maximum cycles, there were no statistically significant differences relating to fatigue resistance for the various abutment systems and this was also not altered by different manufacturer-recommended tightening torques.47,49 When focusing on the F50 value, Ribeiro et al. demonstrated superior fatigue resistance for external hexagonal implant–abutment systems (F50: 53.5 6 7.8 N), with no statistically significant differences between the conical (F50: 44 6 2.49 N) and internal hexagonal (F50: 45 6 3.40 N) interfaces.48 Failure of the abutments was system-dependent and occurred primarily in the region of the weakest point, the screws, respectively, the threaded 569 parts, or between the threaded or unthreaded parts of the abutments.48,49,51 Bending moment/ maximal load resistance. All four trials focused on bending moment/maximal load resistance of the implant–abutment connection used similar methods.53–56 Coppede et al. measured maximum deformation force (MDF) and fracture force (FF) of the specimens under a compressive load delivered at 45! from the vertical (500 gkf load cell with 1 mm/min dislocation) for internal conical (one-piece) and internal hexagonal (two-piece) implant– abutment connection systems.53 Higher MDF values were demonstrated for the internal conical implant–abutment connection (90.58 6 6.72 kgf) as compared to the internal hexagonal connection with a two-piece abutment (83.72 6 4.94 kgf). Fractures only occurred in the internal hexagonal group at the weakest point; the threaded part of the screw. No fractures were detected in the abutments or implants.53 Norton et al. studied the resistance of different internal conical implant–abutment connections and systems (ITI and Astratech with solid abutments), an external hexagonal connection (Branemark, Nobel Biocare) and an internal hexagonal connection with cone and a two-piece abutment (Astratech).54–56 The findings were published in three papers where comparable loading approaches were used. High load tests were run with increasing force and at a constant velocity of 1 mm/min until the applied load caused a failure of the unit or maximal load was achieved. Plastic bending (PB) and maximal bending (Mb) moments were measured and compared between systems and connections. The internal conical implant–abutment connection with R ) and the internal hexagonal a one-piece abutment (AstratechV R) connection with cone and a two-piece abutment (AstratechV had the highest resistance to bending forces. No statistically significant differences were observed for any of the parameters studied in any of the implant systems.55 In comparing different internal conical one-piece abutment systems R and ITIV R ) it was shown that there was signifi(AstratechV R cantly higher resistance to bending forces for the AstratechV implant–abutment connection system54 as compared to the R ), with an ITI system. The Branemark system (Nobel BiocareV external hexagonal connection demonstrated the least resistance (Norton 1997) to bending. Another study62 also documented significantly higher resistance to bending forces for systems using internal conical implant–abutment connections in comparison to those using internal octagonal connections.62 Tightening/loosening torque and cold welding. Six trials studied changes in preload, specifically tightening torque loss or gain of the implant–abutment system.57–62 The principle objectives were to assess the changes in torque after initial tightening and how this was influenced by the following: (1) Increased/decreased initial tightening torque,60 (2) Repeated tightening and removal cycles,58,59,62 and (3) Fatigue loading.57,58,61 Two investigations addressed seal performance, whereas others were focused on stress/load performance, particularly dealing with load fatigue performance of the implant–abutment unit.3,36,50 Ricomini Filho 570 SCHMITT ET AL. et al. and Park et al. documented torque loss following initial tightening of the abutment to the implant but without loading, and this was done with several internal conical and external hexagonal implant–abutment connections.3,57 Ding et al. showed that there was initial loss of torque after tightening; however, this loss was significantly less in the internal conical group in comparison to the internal octagonal group.62 As well, Norton et al. documented no cold welding measuring on the removal torque for ITI and Astratech Morse Taper implant–abutment connection systems with applied torque values between 20 and 40 Ncm. Higher insertion torque values (>100 Ncm) increased the rate of cold welding, but also the rate of fractures.60 The environment (dry and wet) did not influence these outcomes.60 Torque loss was also measured as a result of multiple consecutive closures using different implant–abutment connections. It was shown that when tightening and removal cycles were increased in number, there were concomitant reductions in the torque forces required for removal of the abutment.58,59 Using this approach, Weiss et al. documented significantly higher maintenance of torque values for both conical frictional or interlocking elements.59 The effect of loading on torque required for abutment removal was studied. This demonstrated that internal conical implant–abutment connection systems had significantly less torque loss compared to internal octagonal connection systems3 as well as external hexagonal57 connection systems. It was also shown that loading can cause cold welding to occur between the implant and abutment in conical systems.3,36,58 Alternatively, it was shown that there was more loss of torque in the conical connection group compared to the external hexagonal or internal hexagonal groups after cycling.61 However, they concluded that the design of the connection was not a significant factor in loss of torque but, rather, the screw design such that the use of a screw with a thick stem and a journal provided the least loss of torque after several cycles or tightening and loosening. Stress/strain distribution. Fourteen studies dealt with stress/strain distribution around dental implants and implant–abutment interfaces. Stress transmission from the implant–abutment interface to peri-implant bone was detected using FEM.6,7,37,63,71 Similar data were shown using photoelastic and strain gauge analysis.65–68,70 FEM was also used to examine peaks of stress distribution at the implant–abutment interface. Four trials evaluated the stresses that occur in the periimplant and interface regions (implant–bone interface)6,7,37,63 and three evaluated only interface stresses, and others tried to mimic occlusal loading and assessed the stresses at three interfaces.2,64,69 One study from the load fatigue performance/resistance subgroup also investigated the effects of the addition of more force to the implant– abutment interface, but this was more or less a secondary research objective.52 Of the papers included here for analysis it was found that only two looked at the influence of the implant–abutment joint design on abutment screw loosening using FEM (which of course would lead to movement at PERFORMANCE OF CONICAL IMPLANT–ABUTMENT CONNECTION SYSTEMS REVIEW ARTICLE the abutment–implant interface).5,69 When photoelastic strain gauge analysis was used to assess peri-implant stresses under different loading conditions, it was demonstrated that internal conical connections did not reduce stresses around implants compared with internal or external hexagonal connections.67,68 Cehreli et al. showed that the strain around Brånemark implants with an external hexagonal connection was lower than around ITI and Astratech implants with an internal conical connection, particularly under vertical loads.65 However, the force distribution around the implants systems was similar and it was concluded that the implant–abutment mating design is not the decisive factor affecting stress and strain magnitudes in a bone model.65 Comparing several conical implant–abutment connection systems (ITI, Bicon, Astratech) with a one piece ITI implant revealed that internal-conical connection implants have similar force transfer characteristics than one-piece implants and the connection may not be the decisive factor influencing stress distribution around implants.66 Pessoa et al. documented lower marginal peri-implant bone stresses around an internal conical connection implant compared with internal and external hexagonal connection implants for osseointegrated implants using FEM.37 Also Quaresma et al. showed lower stresses in bone in the conical group compared to the internal hexagonal group.6 Additionally, Lin et al. and Hansson et al. reported that conical implant–abutment connection systems performed better as a force transmission system than internal hexagonal and external flat top systems. This resulted in reduced peak stresses and force transmission to the marginal and apical peri-implant bone regions.7,63 Concerning stress distribution at the implant–abutment interface, several groups documented higher stresses when external hexagonal implant–abutment connection systems were used as compared to when internal conical and internal hexagonal connection systems were utilized.2,37 Additionally, with the use of conical-connection systems there was even more stability of the abutment, also with the smallest microgap in comparison to external and internal hexagonal connection systems.2,5,37,71 Rotational abutment movements and microgap formation were shown most often with implant systems using the external hexagonal connection system.2,5 In vivo Animal studies. This part of the review included five studies. Weng at al. compared radiographic marginal bone level changes around conical and nonconical implant–abutment connection systems for submerged and nonsubmerged implants. Marginal bone level changes were statistically significant, with less bone loss around conical connections of submerged and nonsubmerged implants.72,73 Berglundh et al. documented similar outcomes for submerged healing implants.74 Other included studies reported either comparable or less nonsignificant marginal bone loss around conical connection implants (Table III).75,76 Human studies. The included studies differ in terms of their implant placement and loading protocols. Two studies JOURNAL OF BIOMEDICAL MATERIALS RESEARCH A | FEB 2014 VOL 102A, ISSUE 2 documented implant success and bone level changes around immediately placed and loaded conical and nonconical implant–abutment connection implants.77,78 Further studies followed delayed implant placement protocols with submerged or nonsubmerged healing and delayed or immediate loading protocols.79,80 Regarding implant survival and success rates of included data revealed that conical and nonconical implant–abutment connection systems do not differ statistically. However, three studies documented less marginal bone level changes for conical connection systems, two out of these with a significant difference.77,78,80 Only one study documented higher marginal bone losses around conical implant–abutment connection systems compared to nonconical ones (Table IV).79 DISCUSSION This review found some relevant in vitro and in vivo evidence for the use of conical implant–abutment connection system as it seems superior to nonconical connection systems. Reviewing the current literature concerning the performance of conical implant–abutment connection systems revealed a large number of comparative studies dealing with in vitro investigations. However, only few studies compared conical with nonconical connection implants in vivo. In vitro data revealed that most systems have a gap smaller than 10 mm.39,45 The smallest gap among all connections showed the Astra implants followed by the Ankylos implants that have conical interface geometry.39,45 Therefore, the conical interface geometry seemed to provide a better fit, but may not completely eliminate the gap between the implant and abutment. One may say that a more import factor is the abutment performance under mechanical stress as abutment movement promotes gap enlargement and bacterial penetration. Whether this has any clinical impact remains questionable. For conical connection systems no rotational abutment movement or microgap enlargement was detected under vertical and oblique occlusal loading.2,5,37,71 External and internal hexagonal connection systems were more susceptible to abutment micromovements.2,5,37,71 Another factor for long-term implant–abutment stability may have the maintenance of torque value between implant and abutment after tightening. Obviously this can prevent abutment screw loosening or movement and also microgap formation. All tested connection systems showed torque loss after initial tightening.53,57,62 Mechanical stress showed impact on torque values. In most cases, conical systems showed either higher resistance to torque loss or resulted in cold welding between implant and abutment.3,36,53,57 No cold welding was reported for systems without cone. Multiple consecutive closing and removal cycles showed impact on torque loss for all connection systems.59,62 With the increasing number of cycles the torque value decreased significantly.59,62 Before final insertion of the superstructure, the number of cycles should be minimized in order to avoid further torque loss. All factors promoting the formation of a microgap between implant and abutment may compromise seal 571 performance. As demonstrated, an absolute overall bacterial seal between implant and abutment cannot be achieved. However, most of the results indicate a statistically higher bacterial seal for conical implant–abutment connections systems.1,4,36,40,41 In order to keep bacterial penetration as low as possible, conical connection systems with small microgaps and resistance against abutment movement should be favored. Abutments should be tightened to the implants according to the manufacturer’s recommendations. To guarantee long-term implant success, the number of mechanical complications under loading must be minimized. The implant–abutment connection may well be regarded as a key point to success. The region and mode of abutment fracture also seems to be system specific but quite comparable between systems. It was documented that fractures usually occur at the weakest point of the construction.48,49,51 It should be recognized then, that it is not only the geometry of the implant–abutment interface that might influence abutment fracture resistance, but other components and design factors as well. These could include the number of components (onepiece or two-piece abutment connections), screw-length and diameter; thread design, material as well as contact area. Outcomes of this review suggest that the literature is inconclusive as to what connection is superior insofar as resistance of fracture of the fixture is concerned after loading. Studies that investigated the effects of maximal bending forces on implant systems suggested that implants with a conical implant/abutment connection system were more resistant to fracture than other designs.53–55,60 This was particularly noteworthy with respect to the one- piece conical abutment connection, which provided greater deformation and fracture resistance to the implant–abutment assembly under oblique compressive loading when compared to internal hexagonal and external hexagonal connection systems.53,56 High stress peaks at the implant–abutment interface, particularly the abutment screw, may also explain how some systems fracture or fail. Given these data it would appear that the geometry of the interface might have an important impact on stress distribution and peak stress formation in and around implants, and effects that transcends mere positioning of the implant and even the condition of the bone into which the fixture has been placed.7 Studies included in this review clearly showed that there were significantly lower stress values in the implant–abutment interface of conical implant systems as compared to external hexagonal connections.2,37,64 However, the stresses were not critical for all connections under loading simulation. Despite the comments above, some authors have concluded that the implant–abutment mating design is not a decisive factor insofar as the effects this might have on the magnitudes of stress and strain bone. In relation to this, for example, it has also been suggested that the diameter of the implant could play an important role insofar as resistance to fracture or failure of integration is concerned.64,65 Regarding study characteristics of the clinical studies (animal and human) included in this review it was found that many different experimental approaches were utilized, particularly in the implant placement and loading 572 SCHMITT ET AL. protocols.77–80 Such variation makes it difficult, albeit not impossible to compare the outcomes of the different investigations to one another. Loss of marginal bone loss was observed for all implant systems regardless of whether the implants had been placed using a submerged or nonsubmerged placement protocol. Similarly the placement of immediate or delayed implants (including early or late loading) had no effect on the loss of marginal bone. However, when marginal bone loss was assessed for implants with conical connection systems versus those with nonconical connection systems, it was shown that there was less bone loss about the former in most cases.72,73,75,77,80 Nevertheless, given the state of the literature in this area it must still be recognized that there are probably several factors that might work in concert or singly that influence marginal heights of bone. However, at the very least it would appear that the conical implant connection system is more favorable insofar as maintenance of marginal bone is concerned. CONCLUSION Within the limitations of the present review the following conclusions were drawn: In vitro " No connection has a 100% bacterial seal. However, evidence showed that conical connection systems seem to be superior in terms of bacterial seal. " Conical implant–abutment connection systems seem more resistant to abutment movement and microgap enlargement under loading. Internal and external hexagonal connection systems seem inferior in terms of abutment movement and microgap formation. " Conical connection systems have higher torque loss resistance than other systems. " Conical connection systems have high resistance to fatigue loading and maximum bending. " Conical connection systems seem to have lower abutment screw stresses than external hexagonal connection systems and are comparable to internal hexagonal systems. The cone compensates high stresses and protects the screw from overloading. " The implant–abutment interface geometry seems to be an influencing factor for stress and strain transmission around the implant. In vivo " Conical and nonconical connection systems are comparable in terms of implant success and survival. " In most cases conical connection systems seem to produce a lower marginal bone loss. ACKNOWLEDGMENTS The first author was supported by grants from the Bavarian Association for Scientific Dentistry, Germany (VfwZ) and Dentsply Friadent. The authors declare that there is no conflict of interest associated with this systematic literature review. PERFORMANCE OF CONICAL IMPLANT–ABUTMENT CONNECTION SYSTEMS REVIEW ARTICLE REFERENCES 1. Tesmer M, Wallet S, Koutouzis T, Lundgren T. Bacterial colonization of the dental implant fixture–abutment interface: An in vitro study. J Periodontol 2009;80:1991–1997. 2. Merz BR, Hunenbart S, Belser UC. Mechanics of the implant–abutment connection: An 8-degree taper compared to a butt joint connection. Int J Oral Maxillofac Implants 2000;15:519–526. 3. Ricomini Filho AP, Fernandes FS, Straioto FG, da Silva WJ, Del Bel Cury AA. Preload loss and bacterial penetration on different implant–abutment connection systems. Braz Dent J2010;21:123– 129. 4. Assenza B, Tripodi D, Scarano A, Perrotti V, Piattelli A, Iezzi G, D’Ercole S. Bacterial leakage in implants with different implant– abutment connections: An in vitro study. J Periodontol 2011. 5. Kitagawa T, Tanimoto Y, Odaki M, Nemoto K, Aida M. Influence of implant/abutment joint designs on abutment screw loosening in a dental implant system. J Biomed Mater Res B Appl Biomater 2005;75:457–463. 6. Quaresma SE, Cury PR, Sendyk WR, Sendyk C. A finite element analysis of two different dental implants: Stress distribution in the prosthesis, abutment, implant, and supporting bone. J Oral Implantol 2008;34:1–6. 7. Lin CL, Chang SH, Chang WJ, Kuo YC. Factorial analysis of variables influencing mechanical characteristics of a single tooth implant placed in the maxilla using finite element analysis and the statistics-based Taguchi method. Eur J Oral Sci 2007;115:408– 416. 8. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg 2010;8:336–341. 9. Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochranehandbook.org. 10. Mulrow C, Langhorne P, Grimshaw J. Integrating heterogeneous pieces of evidence in systematic reviews. Ann Intern Med 1997;127:989–995. 11. Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: Synthesis of best evidence for clinical decisions. Ann Intern Med 1997;126:376–380. 12. Rack A, Rack T, Stiller M, Riesemeier H, Zabler S, Nelson K. In vitro synchrotron-based radiography of micro-gap formation at the implant–abutment interface of two-piece dental implants. J Synchrotron Radiat 2010;17:289–294. 13. Hansson S. A conical implant–abutment interface at the level of the marginal bone improves the distribution of stresses in the supporting bone. An axisymmetric finite element analysis. Clin Oral Implants Res 2003;14:286–293. 14. Zielak JC, Rorbacker M, Gomes R, Yamashita C, Gonzaga CC, Giovanni AF. In vitro evaluation of the removal force of abutments in frictional dental implants. J Oral Implantol 2011;37:519–523. 15. Deconto MA, Salvoni AD, Wassall T. In vitro microbiological bacterial seal analysis of the implant/abutment connection in morse taper implants: A comparative study between 2 abutments. Implant Dent 2010;19:158–166. 16. Dibart S, Warbington M, Su MF, Skobe Z. In vitro evaluation of the implant–abutment bacterial seal: The locking taper system. Int J Oral Maxillofac Implants 2005;20:732–737. 17. Broggini N, McManus LM, Hermann JS, Medina R, Schenk RK, Buser D, Cochran DL. Peri-implant inflammation defined by the implant–abutment interface. J Dent Res 2006;85:473–478. 18. Piattelli A, Vrespa G, Petrone G, Iezzi G, Annibali S, Scarano A. Role of the microgap between implant and abutment: A retrospective histologic evaluation in monkeys. J Periodontol 2003;74:346–352. 19. Hermann JS, Schoolfield JD, Schenk RK, Buser D, Cochran DL. Influence of the size of the microgap on crestal bone changes around titanium implants. A histometric evaluation of unloaded non-submerged implants in the canine mandible. J Periodontol 2001;72:1372–1383. 20. Dailey B, Jordan L, Blind O, Tavernier B. Axial displacement of abutments into implants and implant replicas, with the tapered JOURNAL OF BIOMEDICAL MATERIALS RESEARCH A | FEB 2014 VOL 102A, ISSUE 2 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. cone-screw internal connection, as a function of tightening torque. Int J Oral Maxillofac Implants 2009;24:251–256. Coelho Goiato M, Pesqueira AA, Falcon-Antenucci RM, Dos Santos DM, Haddad MF, Bannwart LC, Moreno A. Stress distribution in implant-supported prosthesis with external and internal implant–abutment connections. Acta Odontol Scand 2012. Bozkaya D, Muftu S. Mechanics of the tapered interference fit in dental implants. J Biomech 2003;36:1649–1658. Bozkaya D, Muftu S. Efficiency considerations for the purely tapered interference fit (TIF) abutments used in dental implants. J Biomech Eng 2004;126:393–401. Bozkaya D, Muftu S. Mechanics of the taper integrated screwed-in (TIS) abutments used in dental implants. J Biomech 2005;38:87– 97. Chu CM, Huang HL, Hsu JT, Fuh LJ. Influences of internal tapered abutment designs on bone stresses around a dental implant: three-dimensional finite element method with statistical evaluation. J Periodontol 2011. Pappalardo S, Milazzo I, Nicoletti G, Baglio O, Blandino G, Scalini L, Mastrangelo F, Tete S. Dental implants with locking taper connection versus screwed connection: microbiologic and scanning electron microscope study. Int J Immunopathol Pharmacol 2007;20(1 Suppl 1):13–17. Mollersten L, Lockowandt P, Linden LA. Comparison of strength and failure mode of seven implant systems: An in vitro test. J Prosthet Dent 1997;78:582–591. Gross M, Abramovich I, Weiss EI. Microleakage at the abutmentimplant interface of osseointegrated implants: A comparative study. Int J Oral Maxillofac Implants 1999;14:94–100. Freitas-Junior AC, Almeida EO, Bonfante EA, Silva NR, Coelho PG. Reliability and failure modes of internal conical dental implant connections. Clin Oral Implants Res 2012. Ho DS, Yeung SC, Zee KY, Curtis B, Hell P, Tumuluri V. Clinical and radiographic evaluation of NobelActive(TM) dental implants. Clin Oral Implants Res 2011. Eliasson A, Blomqvist F, Wennerberg A, Johansson A. A retrospective analysis of early and delayed loading of full-arch mandibular prostheses using three different implant systems: Clinical results with up to 5 years of loading. Clin Implant Dent Relat Res 2009;11:134–148. Astrand P, Engquist B, Dahlgren S, Grondahl K, Engquist E, Feldmann H. Astra Tech and Branemark system implants: A 5-year prospective study of marginal bone reactions. Clin Oral Implants Res 2004;15:413–420. Puchades-Roman L, Palmer RM, Palmer PJ, Howe LC, Ide M, Wilson RF. A clinical, radiographic, and microbiologic comparison of Astra Tech and Branemark single tooth implants. Clin Implant Dent Relat Res 2000;2:78–84. Jacobs R, Pittayapat P, van Steenberghe D, De Mars G, Gijbels F, Van Der Donck A, Li L, Liang X, Van Assche N, Quirynen M, Naert I. A split-mouth comparative study up to 16 years of two screwshaped titanium implant systems. J Clin Periodontol 2010;37:1119–1127. Engquist B, Astrand P, Dahlgren S, Engquist E, Feldmann H, Grondahl K. Marginal bone reaction to oral implants: a prospective comparative study of Astra Tech and Branemark System implants. Clin Oral Implants Res 2002;13:30–37. Koutouzis T, Wallet S, Calderon N, Lundgren T. Bacterial colonization of the implant–abutment interface using an in vitro dynamic loading model. J Periodontol 2011;82:613–618. Pessoa RS, Muraru L, Junior EM, Vaz LG, Sloten JV, Duyck J, Jaecques SV. Influence of implant connection type on the biomechanical environment of immediately placed implants—CT-based nonlinear, three-dimensional finite element analysis. Clin Implant Dent Relat Res 2010;12:219–234. Aloise JP, Curcio R, Laporta MZ, Rossi L, da Silva AM, Rapoport A. Microbial leakage through the implant–abutment interface of Morse taper implants in vitro. Clin Oral Implants Res 2010;21:328– 335. Jansen VK, Conrads G, Richter EJ. Microbial leakage and marginal fit of the implant–abutment interface. Int J Oral Maxillofac Implants 1997;12:527–540. 573 40. Teixeira W, Ribeiro RF, Sato S, Pedrazzi V. Microleakage into and from two-stage implants: An in vitro comparative study. Int J Oral Maxillofac Implants 2011;26:56–62. 41. Tripodi D, Vantaggiato G, Scarano A, Perrotti V, Piattelli A, Iezzi G, D’Ercole S. An in vitro investigation concerning the bacterial leakage at implants with internal hexagon and Morse taper implant–abutment connections. Implant Dent 2012;21:335–339. 42. Harder S, Dimaczek B, Acil Y, Terheyden H, Freitag-Wolf S, Kern M. Molecular leakage at implant–abutment connection—in vitro investigation of tightness of internal conical implant–abutment connections against endotoxin penetration. Clin Oral Investig 2010;14:427–432. 43. do Nascimento C, Miani PK, Pedrazzi V, Goncalves RB, Ribeiro RF, Faria AC, Macedo AP, de Albuquerque RF, Jr. Leakage of saliva through the implant–abutment interface: In vitro evaluation of three different implant connections under unloaded and loaded conditions. Int J Oral Maxillofac Implants 2012;27:551–560. 44. Coelho PG, Sudack P, Suzuki M, Kurtz KS, Romanos GE, Silva NR. In vitro evaluation of the implant abutment connection sealing capability of different implant systems. J Oral Rehabil 2008;35:917–924. 45. Baixe S, Fauxpoint G, Arntz Y, Etienne O. Microgap between zirconia abutments and titanium implants. Int J Oral Maxillofac Implants 2010;25:455–460. 46. McGuff HS, Heim-Hall J, Holsinger FC, Jones AA, O’Dell DS, Hafemeister AC. Maxillary osteosarcoma associated with a dental implant: report of a case and review of the literature regarding implant-related sarcomas. J Am Dent Assoc 2008;139:1052–1059. 47. Seetoh YL, Tan KB, Chua EK, Quek HC, Nicholls JI. Load fatigue performance of conical implant–abutment connections. Int J Oral Maxillofac Implants 2011;26:797–806. 48. Ribeiro CG, Maia ML, Scherrer SS, Cardoso AC, Wiskott HW. Resistance of three implant–abutment interfaces to fatigue testing. J Appl Oral Sci. Revista FOB. 2011;19:413–420. 49. Quek HC, Tan KB, Nicholls JI. Load fatigue performance of four implant–abutment interface designs: Effect of torque level and implant system. Int J Oral Maxillofac Implants 2008;23:253–262. 50. Cehreli MC, Akca K, Iplikcioglu H, Sahin S. Dynamic fatigue resistance of implant–abutment junction in an internally notched morse-taper oral implant: Influence of abutment design. Clin Oral Implants Res 2004;15:459–465. 51. Khraisat A, Stegaroiu R, Nomura S, Miyakawa O. Fatigue resistance of two implant/abutment joint designs. J Prosthet Dent 2002;88:604–610. 52. Perriard J, Wiskott WA, Mellal A, Scherrer SS, Botsis J, Belser UC. Fatigue resistance of ITI implant–abutment connectors—A comparison of the standard cone with a novel internally keyed design. Clin Oral Implants Res 2002;13:542–549. 53. Coppede AR, Bersani E, de Mattos Mda G, Rodrigues RC, Sartori IA, Ribeiro RF. Fracture resistance of the implant–abutment connection in implants with internal hex and internal conical connections under oblique compressive loading: An in vitro study. IntJ Prosthodont 2009;22:283–286. 54. Norton MR. In vitro evaluation of the strength of the conical implant-to-abutment joint in two commercially available implant systems. J Prosthet Dent 2000;83:567–571. 55. Norton MR. An in vitro evaluation of the strength of a 1-piece and 2-piece conical abutment joint in implant design. Clin Oral Implants Res 2000;11:458–464. 56. Norton MR. An in vitro evaluation of the strength of an internal conical interface compared to a butt joint interface in implant design. Clin Oral Implants Res 1997;8:290–298. 57. Park JK, Choi JU, Jeon YC, Choi KS, Jeong CM. Effects of abutment screw coating on implant preload. J Prosthodont 2010;19:458–464. 58. Ricciardi Coppede A, de Mattos Mda G, Rodrigues RC, Ribeiro RF. Effect of repeated torque/mechanical loading cycles on two different abutment types in implants with internal tapered connections: An in vitro study. Clin Oral Implants Res 2009;20:624–632. 59. Weiss EI, Kozak D, Gross MD. Effect of repeated closures on opening torque values in seven abutment-implant systems. J Prosthet Dent 2000;84:194–199. 574 SCHMITT ET AL. 60. Norton MR. Assessment of cold welding properties of the internal conical interface of two commercially available implant systems. J Prosthet Dent 1999;81:159–166. 61. Piermatti J, Yousef H, Luke A, Mahevich R, Weiner S. An in vitro analysis of implant screw torque loss with external hex and internal connection implant systems. Implant Dent 2006;15:427–435. 62. Ding TA, Woody RD, Higginbottom FL, Miller BH. Evaluation of the ITI Morse taper implant/abutment design with an internal modification. Int J Oral Maxillofac Implants 2003;18:865–872. 63. Hansson S. Implant–abutment interface: Biomechanical study of flat top versus conical. Clin Implant Dent Relat Res 2000;2:33–41. 64. Alkan I, Sertgoz A, Ekici B. Influence of occlusal forces on stress distribution in preloaded dental implant screws. J Prosthet Dent 2004;91:319–325. 65. Cehreli M, Duyck J, De Cooman M, Puers R, Naert I. Implant design and interface force transfer. A photoelastic and straingauge analysis. Clin Oral Implants Res 2004;15:249–257. 66. Akca K, Cehreli MC. A photoelastic and strain-gauge analysis of interface force transmission of internal-cone implants. Int J Periodont Restorative Dent 2008;28:391–399. 67. Bernardes SR, de Araujo CA, Neto AJ, Simamoto Junior P, das Neves FD. Photoelastic analysis of stress patterns from different implant–abutment interfaces. Int J Oral Maxillofac Implants 2009;24:781–789. 68. Nishioka RS, de Vasconcellos LG, de Melo Nishioka GN. Comparative strain gauge analysis of external and internal hexagon, Morse taper, and influence of straight and offset implant configuration. Implant Dent 2011;20:e24–e32. 69. Saidin S, Abdul Kadir MR, Sulaiman E, Abu Kasim NH. Effects of different implant– connections on micromotion and stress distribution: Prediction of microgap formation. J Dent 2012;40:467–474. 70. Pellizzer EP, Carli RI, Falcon-Antenucci RM, Verri FR, Goiato MC, Villa LM. Photoelastic analysis of stress distribution with different implant systems. J Oral Implantol 2011. 71. Yamanishi Y, Yamaguchi S, Imazato S, Nakano T, Yatani H. Influences of implant neck design and implant–abutment joint type on periimplant bone stress and abutment micromovement: Three-dimensional finite element analysis. Dent Mater 2012;28:1126–1133. 72. Weng D, Nagata MJ, Leite CM, de Melo LG, Bosco AF. Influence of microgap location and configuration on radiographic bone loss in nonsubmerged implants: An experimental study in dogs. Int J Prosthodont 2011;24:445–452. 73. Weng D, Nagata MJ, Bosco AF, de Melo LG. Influence of microgap location and configuration on radiographic bone loss around submerged implants: An experimental study in dogs. Int J Oral Maxillofac Implants 2011;26:941–946. 74. Berglundh T, Abrahamsson I, Lindhe J. Bone reactions to longstanding functional load at implants: An experimental study in dogs. J Clin Periodontol 2005;32:925–932. 75. Abrahamsson I, Berglundh T, Wennstrom J, Lindhe J. The periimplant hard and soft tissues at different implant systems. A comparative study in the dog. Clin Oral Implants Res 1996;7:212–219. 76. Abrahamsson I, Berglundh T, Lindhe J. Soft tissue response to plaque formation at different implant systems. A comparative study in the dog. Clin Oral Implants Res 1998;9:73–79. 77. Pieri F, Aldini NN, Marchetti C, Corinaldesi G. Influence of implant– abutment interface design on bone and soft tissue levels around immediately placed and restored single-tooth implants: A randomized controlled clinical trial. Int J Oral Maxillofac Implants 2011;26:169– 178. 78. Crespi R, Cappare P, Gherlone E. Radiographic evaluation of marginal bone levels around platform-switched and non-platformswitched implants used in an immediate loading protocol. Int J Oral Maxillofac Implants 2009;24:920–926. 79. Kielbassa AM, Martinez-de Fuentes R, Goldstein M, Arnhart C, Barlattani A, Jackowski J, Knauf M, Lorenzoni M, Maiorana C, MericskeStern R, Rompen E, Sanz M. Randomized controlled trial comparing a variable-thread novel tapered and a standard tapered implant: Interim one-year results. J Prosthet Dent 2009;101:293–305. 80. Bilhan H, Kutay O, Arat S, Cekici A, Cehreli MC. Astra Tech, Branemark, and ITI implants in the rehabilitation of partial edentulism: Two-year results. Implant Dent 2010;19:437446. PERFORMANCE OF CONICAL IMPLANT–ABUTMENT CONNECTION SYSTEMS