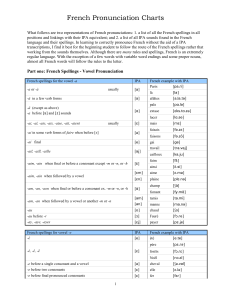
CASE SERIES Contralateral Anterior Interhemispheric Approach to Medial Frontal Arteriovenous Malformations: Surgical Technique and Results Ahmad Hafez, MD∗ ‡§ BACKGROUND: Medial frontal arteriovenous malformations (AVMs) require opening the interhemispheric fissure and are traditionally accessed through an ipsilateral anterior interhemispheric approach (IAIA). The contralateral anterior interhemispheric approach (CAIA) flips the positioning with the midline still positioned horizontally for gravity retraction, but with the AVM on the upside and the approach from the contralateral, dependent side. OBJECTIVE: To determine whether the perpendicular angle of attack associated with the IAIA converts to a more favorable parallel angle of attack with the CAIA. METHODS: The CAIA was used in 6 patients with medial frontal AVMs. Patients and AVM characteristics, as well as pre- and postoperative clinical and radiographic data, were reviewed retrospectively. RESULTS: Four patients presented with unruptured AVMs, with 5 AVMs in the dominant, left hemisphere. The lateral margin was off-midline in all cases, and average nidus size was 2.3 cm. All AVMs were resected completely, as confirmed by postoperative catheter angiography. All patients had good neurological outcomes, with either stable or improved modified Rankin Scores at last follow-up. CONCLUSIONS: This study demonstrates that the CAIA is a safe alternative to the IAIA for medial frontal AVMs that extend 2 cm or more off-midline into the deep frontal white matter. The CAIA aligns the axis of the AVM nidus parallel to the exposure trajectory, brings its margins in view for circumferential dissection, allows gravity to deliver the nidus into the interhemispheric fissure, and facilitates exposure of the lateral margin for the final dissection, all without resecting or retracting adjacent normal cortex. Kunal P. Raygor, MD∗§ Michael T. Lawton, MD∗ ∗ Department of Neurological Surgery, University of California, San Francisco, San Francisco, California; ‡ Department of Neurosurgery, Helsinki University Central Hospital, Helsinki, Finland § These authors contributed equally to this work. Correspondence: Michael T. Lawton, MD, University of California San Francisco, Department of Neurosurgery, 505 Parnassus Avenue, M780, San Francisco, CA 94143. E-mail: [email protected] Received, July 17, 2016. Accepted, January 10, 2017. C 2017 by the Congress of Copyright Neurological Surgeons KEY WORDS: Arteriovenous malformation, Medial frontal lobe, Contralateral anterior interhemispheric approach, Ipsilateral anterior interhemispheric approach, AVM resection Operative Neurosurgery 00:1–9, 2017 I n a previous review of 132 patients with parafalcine and midline arteriovenous malformations (AVMs), we exposed all AVMs with a bilateral craniotomy across the superior sagittal sinus (SSS) and a wide opening of the interhemispheric fissure to access deep feeders from the anterior and posterior cerebral arteries, as well as important draining veins on the medial hemisphere.1 The bilateral ABBREVIATIONS: AVM, arteriovenous malformations; IAIA, ipsilateral anterior interhemispheric approach; CAIA, contralateral anterior interhemispheric approach; SSS, superior sagittal sinus; mRS, modified Rankin Score; MRI, magnetic resonance imaging; AIFA, anterior inferior frontal artery OPERATIVE NEUROSURGERY DOI: 10.1093/ons/opx004 craniotomy completely exposed the midline to optimize access and visualization. In all of these operations, the AVM was approached ipsilaterally in order to keep the anatomy completely in view and have full access to the nidus. With many medial frontal AVMs, an ipsilateral anterior interhemispheric approach (IAIA) was used with the midline positioned horizontally to allow gravity to retract the frontal lobe ipsilateral to the AVM, creating a deep and perpendicular perspective with limited access to the margins of the AVM other than that on the medial surface. The AVM was dissected up from the sagging frontal lobe, drawing the AVM medially into the interhemispheric fissure with restricted access to the lateral and deep margins that often receive supply from VOLUME 00 | NUMBER 0 | 2017 | 1 HAFEZ ET AL challenging lenticulostriate or insular perforating arteries travelling through deep frontal white matter. In contrast to the IAIA, the contralateral anterior interhemispheric approach (CAIA) flips the positioning, still with the midline positioned horizontally for gravity retraction, but with the AVM on the upside and the approach from the contralateral, dependent side. This geometry provides the same exposure of the medial hemisphere and the AVM’s midline anatomy, but with circumferential dissection around the nidus, gravity delivers the nidus into the interhemispheric fissure and improves access to those treacherous lateral and deep margins. In addition, the crossing midline trajectory requires less retraction and/or resection of adjacent cortex than the IAIA. Disadvantages of this CAIA include involvement of the unaffected contralateral hemisphere, the falx as a barrier to access, a deeper reach to the lateral border, and a general concern that intraoperative bleeding would be more difficult to manage from the contralateral side. With increasing AVM experience, the disadvantages and concerns associated with the CAIA seemed manageable and offset by advantages with larger medial frontal AVMs. Therefore, the CAIA was performed in 6 AVM patients with the hypothesis that the perpendicular angle of attack associated with the IAIA would convert to a more favorable parallel angle of attack with the CAIA, with less retraction, improved working angles, and no measurable increase in risk. METHODS Patients After obtaining Institutional Review Board approval, we performed a retrospective review of relevant electronic health records for surgeries performed by the senior author between January 1998 and March 2016. Six patients underwent resection of their medial frontal AVMs via the CAIA. The decision to use this technique was made after reviewing preoperative imaging. Pre- and postoperative clinical and radiographic data including age, gender, presenting symptoms, AVM laterality, maximum nidus size, Spetzler-Martin grade,2 Supplementary grade,3 feeding arteries, draining veins, involved cortical structures, presence or absence of preoperative embolization, and functional status as measured by modified Rankin Score (mRS) were obtained and reviewed. Surgical Technique for the CAIA The patient is positioned supine with the ipsilateral shoulder bolstered. The head is turned 90 degrees to orient the midline horizontally (parallel to the floor), and the head is angled upward 60 degrees. The ipsilateral hemisphere containing the AVM faces the ceiling (upside) and gravity retracts the contralateral hemisphere. The head is fixed in the Mayfield head holder, and surgical neuronavigation is registered to precisely locate the AVM and plan the craniotomy to avoid bridging veins. No lumbar drain is used. After making a semicircular scalp incision, the scalp flap is reflected anteriorly. A single burr hole is drilled approximately 2 cm lateral to the midline along the posterior aspect of the craniotomy, after which a bifrontal craniotomy flap is elevated that 2 | VOLUME 00 | NUMBER 0 | 2017 crosses the midline and expose the SSS (Figure 1). More than two-thirds of the craniotomy flap is on the side contralateral to the AVM. The dura is opened in a curvilinear flap based along the SSS and tacked up medially (Figure 1). Using microsurgical technique, the interhemispheric fissure is opened widely and the hemisphere contralateral to the AVM is covered with protective patties. Unlike the IAIA (Figures 1A-1E), no mechanical brain retraction is needed in the contralateral approach (Figures 1F1J). Anteriorly located AVMs may lie beneath the free edge of the falx where it is thin, whereas posteriorly located AVMs may lie behind the falx and require the opening of a falcine window. The trajectory of the subarachnoid dissection begins in the contralateral interhemispheric fissure and ends ipsilateral to the AVM after crossing the falx. Feeding arteries and draining veins may adhere to the falx on the ipsilateral side, requiring falcine incisions made layer by layer with countertraction away from the nidus, as well as neuronavigation. Major arterial feeders are identified, occluding terminal feeders and skeletonizing en passage arteries. After dearterializing the medial face, the nidus is dissected circumferentially, exposing the lateral margin of the nidus and mobilizing it into the interhemispheric fissure to gain clear visualization of the plane where deep perforating arteries supply the AVM. The draining veins are cauterized and divided after they darken, allowing the nidus to be removed en bloc. RESULTS Between January 1, 1998 and March 31, 2016, the senior author (MTL) microsurgically treated a total of 725 AVMs, of which 26 (3.6%) were located in the medial frontal lobe. Six recent patients underwent AVM resection using the CAIA (Table). The median age was 40 (range, 12-55), and patients were evenly divided by sex. Two patients presented with intracranial hemorrhage, 2 with headache alone, 1 with seizures, and 1 with blurry vision and papilledema. All patients were right-handed, and 5 had AVMs in the left hemisphere. The average AVM diameter was 2.3 cm, and 2 patients had a nidus greater than 3 cm in diameter. One AVM involved eloquent motor cortex within the paracentral lobule. Interestingly, deep venous drainage via anterior or posterior caudate veins to the thalamostriate and internal cerebral veins was found in 3 patients. Five patients had Spetzler-Martin grade II AVMs, whereas Supplementary grades were more variable. No surgical complications were observed. All AVMs were resected completely, as confirmed by postoperative catheter angiography. All patients had good neurological outcomes (mRS scores 0 or 1), with either stable or improved mRS at last followup. The median duration of follow-up was 73 days (range 39-190 days). Illustrative Cases Case 3 A 49-year-old female was diagnosed with a left medial frontal AVM on magnetic resonance imaging (MRI; Figure 2A) after papilledema was found during a routine exam for eye-glasses. She had noted a few years of worsening blurry vision at that time. www.operativeneurosurgery-online.com CONTRALATERAL ANTERIOR INTERHEMISPHERIC APPROACH FOR MEDIAL FRONTAL AVMS OPERATIVE NEUROSURGERY VOLUME 00 | NUMBER 0 | 2017 | 3 HAFEZ ET AL FIGURE 1. Overview of surgical technique with the IAIA and CAIA. A, For the IAIA, the head is positioned with midline horizontal (scalp incision (dashed line), burr hole (white circle), and bifrontal craniotomy (solid line)), the right AVM on the down side, and B the neck angled 45 degrees upwards. C, In the coronal cross-sectional view, the neurosurgeon’s point of view is almost vertically downward towards the dependent medial hemisphere, which makes the lateral margin (green star) inaccessible without additional retraction or tissue resection along the dashed arrow. D, Surgeon’s view demonstrates excellent access to the medial AVM margin and feeders from the right pericallosal artery (PcaA), but E the nidus must be mobilized into the interhemispheric fissure against gravity and the frontal lobe must be retracted or some tissue resected to circumdissect the lateral margin (dashed arrow, coronal cross-sectional view). F, For the CAIA, the head is positioned with midline horizontal (scalp incision (dashed line), burr hole (white circle), and bifrontal craniotomy (solid line)), the left AVM on the up side, and G the neck angled 60 degrees upwards. H, In the coronal cross-sectional view, the neurosurgeon’s point of view is almost horizontal over the dependent medial hemisphere and across the interhemispheric fissure, which makes the lateral margin (green star) accessible without additional retraction or tissue resection. I, Surgeon’s view demonstrates excellent access to the medial AVM margin and feeders from the left pericallosal artery after incising and resecting a piece of the falx (see inset). J, The nidus falls into the interhemispheric fissure with gravity, providing a direct view of the lateral margin, thereby facilitating circumdissection of the AVM’s most difficult margin (dashed arrow, coronal cross-sectional view). Abbreviations: SSS = superior sagittal sinus; ISS = inferior sagittal sinus; CC = corpus callosum. TABLE. Patient and AVM Characteristics for Patients Undergoing the CAIA to AVM Resection Case Age (yrs), sex 1 15, M 2 3 48, F 49, F 4 12, M 5 6 55, M 32, F Presenting symptoms Side of AVM Nidus size (cm) SM grade Supp grade Feeding arteries Draining vein(s) Structures involved Preop Embo Preop mRS Postop EBL mRS (mL) HA, seizure, ICH HA Blurry vision and papilledema Prior ICH, recovered Left 1.7 2 1 CmaA AntCaudV Cing Crtx 0 1 1 400 Left Left 1 2.5 2 2 4 4 PcaA, CmaA AIFA, FrPolA, CmaA, PcaA AntCaudV MedFrontV, PcaV Cing Crtx Cing Crtx 0 1 1 1 0 0 300 500 Left 1.7 3 2 PcaA, ParaCenA PostCaudV 0 0 0 200 Seizure HA Right Left 3.1 3.5 2 2 4 3 FrPolA, CmaA MedFrontV AIFA, PcaA MedFrontV Cing Crtx, Paracentral lobule Cing Crtx Cing Crtx 0 1 1 1 1 0 500 300 ABBREVIATIONS: AIFA, anterior internal frontal artery; AntCaudV, anterior caudate vein; Cing Crtx, cingulate cortex; CmaA, callosomarginal artery; EBL, estimated blood loss; Embo, embolization; FrPolA, frontopolar artery; HA, headache; ICH, intracranial hemorrhage; MedFrontV, medial frontal vein; mL, milliliter; mRS, modified Rankin Score; ParaCenA, paracentral artery; PcaA, pericallosal artery; PcaV, pericallosal vein; PostCaudV, posterior caudate vein; SM, Spetzler-Martin; SSS, superior sagittal sinus; Supp, Supplementary. Preoperative angiogram demonstrated feeders from the anterior inferior frontal artery (AIFA), frontopolar, callosomarginal, and pericallosal arteries, and venous drainage through medial frontal and pericallosal veins (Figures 2B and 2C). The largest feeding artery from AIFA was embolized preoperatively, as was an intranidal aneurysm. The AVM was resected through the CAIA. After opening the interhemispheric fissure widely, subarachnoid dissection was carried down to the corpus callosum and the falx was divided (Figure 2D) to provide view of the 4 trunks feeding the AVM. The trunk that had been completely embolized was clip-occluded, cauterized, and divided; the others were skeletonized to preserve en passage arteries (Figure 2E). A plane of dissection was developed anteriorly, posteriorly, and laterally around the nidus, allowing the nidus to be pulled into the interhemispheric fissure. The draining veins were divided and the nidus was removed (Figure 2F). Postoperatively, the patient did well, with slight improvement in her vision and no new 4 | VOLUME 00 | NUMBER 0 | 2017 neurological deficits. Postoperative angiogram showed no residual AVM nidus (Figures 2G and 2H). Case 6 A 32-year-old female was seen in clinic after an MRI performed for persistent headaches revealed a left medial frontal AVM (Figure 3A). Initial angiogram demonstrated AIFA and pericallosal feeding arteries and superficial venous drainage from anterior medial frontal veins (Figures 3B and 3C). She underwent preoperative embolization of the 2 major feeding arteries. Intraoperatively, the anterior cerebral artery was followed along its course to get around the dilated draining veins (Figure 3D). The AVM was dissected circumferentially to occlude all feeders, and the draining vein was divided to remove the nidus (Figures 3E3G). Postoperatively, the patient did well, with resolution of her frontal headaches and no new neurological deficits. Postoperative www.operativeneurosurgery-online.com CONTRALATERAL ANTERIOR INTERHEMISPHERIC APPROACH FOR MEDIAL FRONTAL AVMS A B C D E G H F FIGURE 2. Pre-, intra-, and postoperative images from Case 3. A, Preoperative axial MRI demonstrating a left medial frontal AVM with multiple dilated veins anterior and posterior to the nidus making an ipsilateral approach treacherous. B, C Preoperative anteroposterior (AP) and lateral digital subtraction angiography (DSA) demonstrating the left medial frontal AVM with its associated arterial feeders and draining veins. D, Intraoperative photo demonstrating division of the falx cerebri. E, Intraoperative photo demonstrating skeletonized feeding arteries. F, Intraoperative photo of the AVM nidus after it is pulled into the contralateral interhemispheric fissure. G, H Postoperative AP and lateral DSA showing no residual arteriovenous (AV) shunting. angiogram showed no residual AVM nidus and preservation of en passage arteries (Figures 3H and 3I). DISCUSSION Contralateral approaches through the interhemispheric fissure are designed for accessing lesions located laterally in or adjacent to the lateral ventricle.4-6 With the contralateral transcallosal approach, the head is positioned with the midline oriented horizontally and the lesion on the upside to allow gravity OPERATIVE NEUROSURGERY to retract the dependent hemisphere and open the interhemispheric fissure without retractor blades.7 A crossing trajectory begins in the anterior interhemispheric fissure contralateral to the lesion but ends in the ventricle ipsilateral to the lesion, thereby maximizing the lateral exposure in the ventricle to comfortably access small AVMs in the ventricular wall, caudate head, and thalamus. Similar advantages in exposure and access result from gravity retraction and a crossing trajectory with the CAIA to lesions in the medial frontal lobe that are not strictly intra- or periventricular. However, this approach has VOLUME 00 | NUMBER 0 | 2017 | 5 HAFEZ ET AL A B C D E F G H I FIGURE 3. Pre-, intra-, and postoperative images from Case 6. A, Preoperative coronal MRI demonstrating a left medial frontal AVM. B, C, Preoperative AP and lateral DSA demonstrating the left medial frontal AVM with its associated arterial feeders and draining veins. D, E, Intraoperative photos demonstrating identification and then cauterization of arterial feeders. F, Intraoperative photo showing removal of the nidus after dividing the draining veins. G, Intraoperative photo showing preservation of en passage arteries. H, I, Postoperative AP and lateral DSA showing no residual AV shunting. not been widely used with AVMs for several reasons. First, an approach from the contralateral side exposes both hemispheres to surgical manipulation and risk of injury. Second, the falx is a midline barrier that conceals AVM anatomy and must be incised or excised to access the lesion. Third, the surgical corridor is deepened with a crossing trajectory. And finally, intraoperative AVM hemorrhage at increased depths and in a hemisphere that is only minimally exposed by the craniotomy might be more dangerous and difficult to control. Therefore, the IAIA has been the predominant approach to medial frontal AVMs.8 6 | VOLUME 00 | NUMBER 0 | 2017 Medial frontal AVMs with a lateral margin that extends 2 cm or more from the midline are difficult to remove safely through the IAIA mainly because the exposure of this lateral margin is poor. When the AVM lies in the dependent hemisphere, the medial aspect of the superior frontal gyrus conceals the AVM’s lateral margin and must be retracted or resected to access this margin. Retraction or brain resection with medial frontal AVMs that are more posterior can damage motor and supplementary motor cortex. Furthermore, AVMs that exceed this 2-cm dimension are more likely to be supplied by lenticulostriate arteries traversing deep frontal white matter that can be difficult to cauterize and www.operativeneurosurgery-online.com CONTRALATERAL ANTERIOR INTERHEMISPHERIC APPROACH FOR MEDIAL FRONTAL AVMS control. Therefore, the lateral margin is the most dangerous one to dissect and has the worst exposure with the IAIA. In addition, medial veins ascending to the SSS typically drain the AVM and can tether the hemisphere, limiting widening of the interhemisphere fissure by gravity retraction. These veins must be preserved for AVM outflow throughout the resection, and an ipsilateral approach places them in the center of the operative field where they become obstacles at risk with the passages and movements of surgical instruments. Therefore, the IAIA is not ideal for these medium-sized, medial frontal AVMs, and the CAIA has important advantages with these particular AVMs that offset its risks. This rationale justified our experience with the CAIA in the 6 patients presented. We did not observe any injury or neurological morbidity in the contralateral hemisphere resulting from our surgical manipulations. Bridging veins in the dependent hemisphere and the risk of venous infarction were our biggest concerns, but these veins were scarce and easily preserved. Neuronavigation was useful in identifying them preoperatively and relocating the craniotomy flap to avoid them. The falx was easily transected to expose the AVM anatomy on the opposite side of the interhemispheric fissure. Although the surgical corridor was deeper than with an IAIA, we did not struggle at the depths of the circumdissection. On the contrary, dissection of the lateral margin was facilitated by a view directly into this plane, a nidus that mobilized medially to widen this plane, and direct access to lenticulostriate perforators as they intersected the lateral and deep margins. Intraoperative AVM hemorrhage was not encountered in this series, but could have been managed easily from the contralateral side. Although this study was a small, highly selected, retrospective surgical series and reflects the experience of a specialized vascular surgeon with a large AVM experience and a well-trained team, results confirm the advantages and safety of the CAIA for medial frontal AVMs. The angle of attack with medial frontal AVMs changes from perpendicular with the IAIA to a parallel with the CAIA. With the IAIA, the axis of the AVM nidus is perpendicular to the trajectory of the exposure, which means that only one side of the AVM is accessed directly and other sides are accessed only with retraction and/or resection of brain and mobilization of the AVM into the interhemispheric fissure. With the CAIA, the axis of the AVM nidus is parallel to the trajectory of the exposure, bringing most of its margins in view and accessing it circumferentially for easy dissection. As circumdissection frees the margins, gravity delivers the AVM into the interhemispheric fissure and widens the lateral margin for the final dissection, which increases the safety of the most treacherous part of the resection. The CAIA requires incisions in the falx with arteriovenous structures hidden on the opposite side. Neuronavigation helps plan these cuts and avoid these hazards. Complete removal of the adjacent piece of falx enhances the exposure. The inferior sagittal sinus can be occluded when it does not drain the AVM, enabling these cuts to extend through the free edge of the falx. The limited transfalcine view requires detailed knowledge of medial frontal lobe surface anatomy.9 The anterior frontal median OPERATIVE NEUROSURGERY cortex is defined: inferiorly by pericallosal arteries separating the cingulate gyrus from the corpus callosum; posteriorly by the paracentral lobule and eloquent motor cortex; anteriorly by the medial aspect of superior frontal gyrus and callosomarginal artery’s branches; and superiorly by the SSS and its associated bridging veins. Orbitofrontal and frontopolar arteries supply anterior medial frontal AVMs, whereas the internal frontal and paracentral arteries supply posterior medial frontal AVMs. Pericallosal and callosomarginal arteries feed cingulate AVMs directly. Venous drainage usually ascends to the SSS through medial frontal veins, but cingulate AVMs often have descending venous drainage to pericallosal veins, inferior sagittal sinus, and occasionally deep to the internal cerebral veins, as seen in our cohort.1 The CAIA is not recommended for all medial frontal AVMs. Small AVMs confined to or just off the midline may not benefit from a contralateral approach. Ruptured AVMs may have associated hematomas and presenting neurological deficits that favor an ipsilateral approach through the hematoma cavity. A medial frontal AVM with a hematoma extending to a cortical surface may be managed best with a transcortical approach, which would require the AVM to be positioned on the upside. CONCLUSION This study demonstrates that the CAIA is a safe alternative to the IAIA for medial frontal AVMs that extend 2 cm or more off the midline into the deep frontal white matter. The CAIA aligns the axis of the AVM nidus parallel to the trajectory of the exposure, bringing most of its margins in view for circumferential dissection, allowing gravity to deliver the AVM into the interhemispheric fissure, and facilitating exposure of the lateral margin for the final dissection, all without resecting or retracting adjacent normal cortex. Disclosures Dr Hafez was awarded the C. Ehrnrooth Fellowship (Fondation de Luxembourg) at the Clinic of Neurosurgical Surgical of the Helsinki University Central Hospital. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. REFERENCES 1. Kim YB, Young WL, Lawton MT, Project UBS. Parafalcine and midline arteriovenous malformations: surgical strategy, techniques, and outcomes. J Neurosurg. 2011;114:984-993. 2. Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483. 3. Lawton MT, Kim H, McCulloch CE, Mikhak B, Young WL. A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery. 2010;66:702-713. 4. Zaidi HA, Chowdhry SA, Nakaji P, Abla AA, Spetzler RF. Contralateral interhemispheric approach to deep-seated cavernous malformations: surgical considerations and clinical outcomes in 31 consecutive cases. Neurosurgery. 2014;75:80-86. 5. Rangel-Castilla L, Spetzler RF. The 6 thalamic regions: surgical approaches to thalamic cavernous malformations, operative results, and clinical outcomes. J Neurosurg. 2015;123:676-685. VOLUME 00 | NUMBER 0 | 2017 | 7 HAFEZ ET AL 6. Bohnstedt BN, Kulwin CG, Shah MV, Cohen-Gadol AA. Posterior interhemispheric transfalcine transprecuneus approach for microsurgical resection of periatrial lesions: indications, technique, and outcomes. J Neurosurg. 2015;123:1045-1054. 7. Lawton MT, Golfinos JG, Spetzler RF. The contralateral transcallosal approach: experience with 32 patients. Neurosurgery. 1996;39:729-735. 8. Stein BM. Arteriovenous malformations of the medial cerebral hemisphere and the limbic system. J Neurosurg. 1984;60:23-31. 9. Rhoton AL Jr. The cerebrum. Anatomy. Neurosurgery. 2007;61:37-119. COMMENTS T he first CNS practical clinic, held in Baltimore, Maryland in October 1987, lasted 8 hours.1 The topic? Surgical positioning. Somewhere along the way, surgical positioning as a topic lost its sex appeal, but it remains an unsung, critical step in the planning of a procedure. It is the first step of performing an operation, and all other phases of a surgery are affected by it. In some cases, the patient is positioned in order to optimize the ergonomics and comfort for the surgeon to reduce fatigue and facilitate careful microsurgery. I have always emphasized this point to trainees. In many cases, positioning reduces or eliminates the need for selfretaining retractors, by letting gravity do the work. The authors of this research have long been proponents of this strategy. The current work emphasizes the degree of nuance that can go into surgical positioning. Lateral positioning with gravity retraction of the cerebral hemisphere for interhemispheric approaches, championed by Spetzler and others, made surgical treatment of deep pathologies near the midline safer and easier. In this report, the authors highlight a useful corollary to the more traditional ipsilateral interhemispheric approach, the contralateral anterior interhemispheric approach (CAIA). As the authors point out, this approach was used in only 6 of 26 cases of medial frontal AVMs. Lesions must be ideally suited for the advantages of this approach (gravity delivery of the malformation, more optimal visualization of the most difficult facets of the AVM) to outweigh the disadvantages (putting 2 hemispheres at risk for injury, and a less comfortable, less ergonomic surgeon position). It is the compromised position of the surgeon that is the main drawback of this approach, in my mind. For long cases and meticulous dissection, I believe surgeon comfort is paramount. A fully adjustable chair and a hand rest can help immensely with this goal. I applaud the authors focus in this research on surgical positioning, and continued refinement of surgical approaches. While microsurgical techniques for cerebrovascular disease are mature, that doesn’t mean they are unable to be improved. There is plenty of room for continued evolution of techniques in both the microsurgical and endovascular realms. Edward Angus McKay Duckworth Houston, Texas 1. CNS Brochure/Personal communication, Harry van Loveren, MD T his small series describes a contralateral interhemispheric approach for medial frontal AVMs. This article is well written and the conclusions are valid. I have used this same approach for similar pathologies and agree with their conclusions and description of the benefits of this approach. A more posterior contralateral interhemispheric approach can also be used for parietal AVMs and deeper lesions including thalamic cavernous malformations with the same benefits cited by the authors. 8 | VOLUME 00 | NUMBER 0 | 2017 The positioning of the patient’s head for this approach is critical and has a number of benefits. The side of the approach is dependent, lateral and parallel to the floor with the vertex tilted upward, giving the surgeon an unencumbered view of the falx with gravity assisting in retraction of the frontal lobe. Once the falx is opened, the pathology is exposed without the need for any retractors. Since our eyes are oriented horizontally, this position also provides superior horizontal visualization of the interhemispheric fissure over the vertical visualization one has with the more traditional supine position of the patient for an interhemispheric approach. One potential risk of this approach is that the dependent frontal lobe falls away from the falx so effectively that draining veins to the superior sagittal sinus may be put on stretch and become injured or thrombosed. These veins must be protected and I have routinely covered them with gelfoam soaked in heparin which will pass through the vein, assist in maintaining their patency and reduce the risk of venous infarction. Daniel L. Barrow Atlanta, Georgia RESPONSE TO REVIEWER COMMENTS Reviewer #2: This is an interesting and well written technical report about the novel description of a contralateral anterior interhemispheric approach (CAIA) to resect frontal medial AVMs instead of the classic ipsilateral anterior interhemispheric approach (IAIA). In 6 patients out of 26 surgically treated medial frontal AVMS the CAIA was used and these 6 patients were further analyzed in this manuscript. The average AVM diameter was 2.3cm and 2 out of 6 patients had a diameter larger than 3cm with mainly low-grade SpetzlerMartin grade (5 patients Spetzler-Martin grade of 2 and 1 patient with grade 3). Outcome was favorable with no surgical complications and good mRS <1 at LFU in all cases, respectively. The authors explain in detail in which scenario a CAIA is useful from their point of view (AVM with a lateral margin that extends 2cm, medially located ascending draining veins being obstacles at risk for a IAIA approach) and why this approach is not recommended in general (exposure of both hemispheres and risk of surgical manipulation and risk of injury, surgical corridor is deepened with a crossing trajectory, intraoperative AVM rupture is difficult to control from a contralateral approach) and applies only for a small selective group of patients. We agree with the authors that this approach is useful in carefully selected patients and we recommend this technical report to be published. However, we were wondering how the authors define inclusion criteria of the lateral margin of 2cm. Is this based on a calculated analysis of all 26 treated medial frontal AVMs or from a subjective point of view? It would be also interesting to have more details on the control group (IAIA) in terms of AVM size, grade and outcome. Response: The inclusion criterion - a lateral margin 2 cm off midline - was not based on a calculated analysis of all medial frontal AVMs, but offered as an empiric rule of thumb. AVMs that are right on the midline or within this 2 cm limit can be easily dissected free and mobilized into the interhemispheric fissure and do not require the steep crossing trajectory of a contralateral approach. We subjectively decided that the advantages of the CAIA were not needed for these smaller midline AVMs and were outweighed by the disadvantages and risks of the CAIA. www.operativeneurosurgery-online.com CONTRALATERAL ANTERIOR INTERHEMISPHERIC APPROACH FOR MEDIAL FRONTAL AVMS Details and surgical results from patients undergoing IAIA were published previously as part of a larger group of midline AVMs (Kim YB, Young WL, Lawton MT, for the UCSF BAVM Study Project: Parafalcine and midline arteriovenous malformations: Surgical strategy, techniques, and outcomes. Journal of Neurosurgery: 114 (4): 984–993, 2011.) This group was not meant to be a control group, and it would be difficult to make meaningful comparisons because the sample sizes in the two groups are small and there is inherent selection bias in the patients who underwent CAIA. We included this total number of medial frontal AVMs only to provide some context for the patients treated with the CAIA, and we do not think that a review of these IAIA patients would add meaningfully to our message. OPERATIVE NEUROSURGERY Reviewer #3: This is a small series that describes a superior contralateral interhemispheric approach for medial frontal AVMs. The article is well written and the conclusions are valid. I have personally used the sam approach as the authors and agree with their conclusions and description of the benefits of this approach. The exposure is excellent after opening the falx and the procedure requires no retraction of the dependent hemisphere. Response: We thank the reviewer for these kind comments and are pleased that his experience agrees with ours. In response to the reviewer’s comments, we revised our illustrations to show the incisions in the falx and the effects of gravity retraction. VOLUME 00 | NUMBER 0 | 2017 | 9