Wound Healing Activity of Acylated Iridoid Glycosides from Scrophularia nodosa
Anuncio

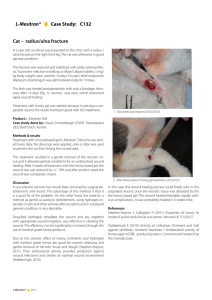
PHYTOTHERAPY RESEARCH Phytother. Res. 16, 33–35 (2002) DOI: 10.1002/ptr.798 SHORT COMMUNICATION Wound Healing Activity of Acylated Iridoid Glycosides from Scrophularia nodosa Philip C. Stevenson1,3, Monique S. J. Simmonds1*, Julia Sampson2, Peter J. Houghton2 and Peter Grice4 1 Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, TW9 3DS, UK Department of Pharmacy, Kings College, University of London, Franklin-Wilkins Building, 150, Stamford Street, London, SE1 8WA, UK Natural Resources Institute, University of Greenwich, Chatham Maritime, ME4 4TB, UK 4 Department of Chemistry, Lensfield Road, Cambridge, CB2 1EW, UK. 2 3 Three acylated iridoid glycosides (E)-6-O-(2@, 4@-diacetyl-3@ -O-p-methoxycinnamoyl)-a-L-rhamnopyranosyl catalpol (scopolioside A) (1), (E)-6-O-(2@-acetyl-3@, 4@-di-O,O-p-methoxycinnamoyl)-a-L-rhamnopyranosyl catalpol (scrophuloside A4) (2) and (E)-6-O-(2@,3@-diacetyl-4@-O-p-methoxycinnamoyl)-a-Lrhamnopyranosyl catalpol (scrovalentinoside) (3) have been isolated from the dried seed pods of Scrophularia nodosa by HPLC. Their structures were determined by 1D and 2D NMR, UV/Vis and mass spectroscopy and by comparison with published data. All three compounds were shown in vitro to stimulate the growth of human dermal fibroblasts. The effect was negatively dose-dependent for 2 and 3 for which fibroblast growth stimulation was highest at 0.78 mg/mL but was not significantly different from the control at 100 mg/mL. The presence of these compounds in the mature seed pods may explain the ethnobotanical use of this plant in Europe for healing wounds. Copyright # 2002 John Wiley & Sons, Ltd. Keywords: wound healing; Scrophularia; iridoid glycosides; figwort. INTRODUCTION The Scrophulariaceae are well known as a source of iridoids and iridoid glycosides many of which are derived from 6-O-a-L-rhamnopyranosyl catalpol where the most common substitutions occur via ester bonds to the rhamnopyranosyl moiety (Miyase and Mimatsu, 1999; Giner et al., 1998; Calis et al., 1988). As part of our studies on the medicinal properties of British and Irish plants we have investigated the common figwort, Scrophularia nodosa, a plant used in parts of Ireland for the treatment of wounds (Grieve, 1992). The aim of the present study was to identify the compounds in the mature seed heads that account for the reported wound healing properties of S. nodosa by evaluating the fibroblast growth stimulating properties using established methods (van Hien et al., 1997). Wound healing involves different phases and processes including inflammation, wound contraction, reepithelialization, tissue remodelling and formation of granulation tissue with angiogenesis (Cherry et al., 1994). Fibroblasts are involved in all of these processes so the human dermal fibroblast (HDF) growth-stimulating activity of plant compounds is an appropriate * Correspondence to: Professor M. S. J. Simmonds, Jodrell Laboratory, Royal Botanic Gardens, Kew, TW9 3DS, UK. E-mail: [email protected] Contract/grant sponsor: Dunhill Medical Trust. Contract/grant sponsor: Rank Foundation. Contract/grant sponsor: Kings College London Research Strategy Fund. Copyright # 2002 John Wiley & Sons, Ltd. technique by which to determine the wound healing properties of the test compounds. MATERIALS AND METHODS Plant material. Mature seed pods of S. nodosa L. were collected by P. Gorman, University of Dublin, from plants growing in County Down, Eire during November 1997. A voucher specimen is deposited in the herbarium of the Department of Botany, University of Dublin. Extraction and isolation. Air-dried mature seed podsof S. nodosa (45 g) were extracted in 1 L of absolute methanol at 45 °C for 1 h and at room temperature for a further 23 h. The methanol extract was filtered, evaporated under reduced pressure and re-dissolved in methanol to a concentration of 1 g plant material/mL. The components of the extract were separated and isolated by HPLC using a Waters system consisting of a 600E controller and 60F pump module, 717 autosampler and 996 photodiode array detector. Filtered (0.45 mM) aliquots (12 150 mL) were injected directly on to a semi-preparative reverse phase C18 Lichrospher column (10 mm i.d. 25 cm; 10 mM particle size). Components of the extract were separated by gradient elution using a binary solvent system consisting of solvent A (methanol) and solvent B (41:10:49, methanol: acetonitrile:water) at 4.70 mL/min. At T = 0.0 min, A = 25% and at T = 25 min A = 100%. Compounds 1, 2 and 3 were detected at 313 nm and eluted at 8.43, 17.59 and 9.52 min, respectively. Compounds were collected Received 16 May 2000 Accepted 27 July 2000 34 P. C. STEVENSON ET AL. Figure 1. Structures of compounds scopolioside A (1), scrophuloside A4 (2) and scrovalentinoside (3). manually with recovery of 43 mg (1), 9 mg (2) and 56 mg (3). Structural determination. NMR spectra for 1, 2 and 3 were recorded in CD3OD on a Bruker DRX 600 instrument at 300K. Spectra were referenced to TMS. Positive ion first order mass spectra of compounds 1, 2 and 3 were recorded (m/z 125–2000) by direct injection into a quadropole ion-trap mass spectrometer (FinniganMatt LCQ) fitted with an ESI source. Fibroblast bioassays. Human dermal fibroblast (HDFs) cells from post auricular surgery were grown to confluence after which they were removed from a culture flask using trypsin/EDTA after washing with phosphate buffer saline (PBS). HDFs were re-suspended in 50 mL of Dulbeccos’ modified eagle medium (DMEM), centrifuged at 2600 rpm for 5 min and seeded in a 96-well sterile microtitre plate at a density of 11 103 cells/well in DMEM containing 10% fetal calf serum (FCS), 0.02% fungizone and 1% penicillin and 2% streptomycin. After 24 h the media was removed by aspiration. Solutions were initially solubilized in water and diluted in DMEM containing 0.5% FBS (0.5% FBS is the maintenance level required for HDF growth) to give a final concentration of 100 mg/mL. Solutions were filtered through a 0.2 mM sterile filter prior to addition to the cells and 1:1 serial dilutions were prepared. Aliquots (200 mL) of each compound, in triplicate, were added to each well. The plates were left to incubate for 3 days. The neutral red assay was used to analyse the effects of the extract on the growth of fibroblasts. Neutral red dye (1.2 mL) was added to 78.8 mL of Hanks’ balanced salt solution (HBSS). This was incubated for 10 min at 37 °C after Copyright # 2002 John Wiley & Sons, Ltd. Figure 2. Effect of compounds scopolioside A (a), scrophuloside A4 (b) and scrovalentinoside (c) on growth of human dermal ®broblasts at different concentrations compared with control cells measured as absorbance at 550 nm using the neutral red assay. * signi®cant growth compared with the FCS (0.5%) control (t-test: p<0.05). which it was centrifuged at 2600 rpm for 5 min, and 100 mL added to each well. The plates were incubated for 2.5 h, the media was tipped off and the cells washed with 100 mL of 1% formic acid followed by 100 mL of 1% acetic acid. The absorbance was read at 550 nm and the values obtained for the solutions were compared with the control (0.5% FCS). RESULTS AND DISCUSSION Semi-preparative HPLC of the crude filtered methanol extract of the dried, milled seed and seed coats of S. nodosa yielded three compounds which were identified as scopolioside A (1), scrophuloside A4 (2) and scrovalentinoside (3) (Fig. 1). Their structures were determined by ID and 2D NMR and mass spectrometry and confirmed by comparison with the published data (Miyase and Mimatsu, 1999; Giner et al., 1998; Calis et al., 1988). Compounds 1, 2 and 3 all stimulated the growth of HDFs compared with a control when tested at <1.0 Phytother. Res. 16, 33–35 (2002) WOUND HEALING AND SCROPHULARIA NODOSA mg/mL. This activity was also greater than that of a positive control in which fibroblasts were allowed to grow in 10% FCS. The use of 10% FCS to indicate HDF growth stimulation is an established comparative technique and provides a suitable positive control (van Hien et al., 1997). Compound 1 was the only one of the three compounds to stimulate HDF growth over the full range (100 mg/mL to 0.78 mg/mL) of concentrations tested (Fig. 2a). Both 2 and 3 showed a negative dose-dependent response, stimulating HDF growth at lower concentrations (Fig. 2b, c). We believe that this is the first report of fibroblast growth stimulating activity for iridoid glycosides and thus the first report indicating that iridoid glycosides may be directly involved in the wound healing process. The mechanism of the activity of 1, 2 and 3 is not presently known so it is not possible to ascertain why a negative dose-dependent effect was recorded for 2 and 3. Scopolioside A has been shown to exhibit antihepatotoxic activity and immunostimulant properties (Garg et 35 al., 1994). Anti-inflammatory activity has been attributed to a mixture of 2 with a 4-O-acetyl-2,3-O-di-p-methoxycinnamoyl isomer (Rios et al., 1991) as well as to less substituted iridoids such as harpagoside (Garcia et al., 1996). Since anti-inflammatory and immuno-stimulant activities are important components for successful wound healing the HDF stimulating properties provides considerable evidence that these highly substituted and closely related iridoid glycosides could be responsible for the wound healing properties of S. nodosa. Acknowledgements The authors thank Dr G.C. Kite for helping obtain MS data, Dr N.C. Veitch for his valuable comments on the manuscript, Dr P. Gorman for provision of plant material and Professor Ley for use of NMR facilities at Cambridge, UK. We thank the Dunhill Medical Trust, Rank Foundation and Kings College London Research Strategy Fund for their financial support. REFERENCES Calis I, Gross G-A, Winkler T, Sticher O. 1988. Isolation and structural elucidation of two highly acylated iridoid diglycosides from Scrophularia scopolii. Planta Med 54: 168±170. Cherry GW, Hughes MA, Kingsnorth AN, Arnold FW. 1994. In Oxford Textbook of Surgery Vol 1, Morris PJ, Malt RA (eds). Oxford University Press: Oxford; 3±23. Garcia A, Fernandez A, Saenz T, Ahumada C. 1996 Antiin¯ammatory effects of different extracts and harpagoside isolated from Scrophularia frutescens. Il Farmaco 51: 443±446. Garg HS, Bhandari SPS, Tripathi SC, et al. 1994 Antihepatotoxic and immunostimulant properties of iridoid glycosides of Scrophularia koelzii. Phytother Res 8: 224±228. Giner RM, Villaba ML, del Carmen Recio M, Manez S, Gray AI, Copyright # 2002 John Wiley & Sons, Ltd. Rios JL. 1998. A new iridoid from Scrophularia auriculata. J Nat Prod 61: 1162±1163. Grieve M. 1992. In, A Modern Herbal. Tiger Books International: London; 313. Miyase T, Mimatsu A. 1999 Acylated iridoid and phenylethanoid glysocides from the aerial parts of Scrophularia nodosa. J Nat Prod 62: 1079±1084. Rios JL, Recio MC, Giner RM, Sanz MJ, Terencio MC, Manez S. 1991. Two new catalpol derivatives from Scrophularia auriculata. Planta Med 57: Suppl 2. Van Hien T, Hughes MA, Cherry WC. 1997. In vitro studies on the antioxidant and growth stimulatory activities of a polyphenolic extract from Cudrania cochichinensis in the treatment of wounds in Vietnam. Wound Rep Regen 5: 159±167. Phytother. Res. 16, 33–35 (2002)