dr marcelo lavarda [Modo de compatibilidad]
Anuncio
![dr marcelo lavarda [Modo de compatibilidad]](http://s2.studylib.es/store/data/008674329_1-2dcc1a0a0eeca4a2ebecb41b33feba2a-768x994.png)
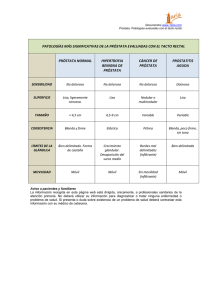
Manejo Sistémico del Cáncer de Próstata Resistente a Hormonoterapia Dr Marcelo Lavarda Servicio de Oncología y Hematología Clínica Sanatorio Allende Córdoba Carcinoma de Próstata Hormono Resistente Terminología Sugerida Andrógeno (hormona) Independiente: AIPC Inexacto: Cáncer de Próstata todavía responde a andrógenos (hormonas) Refractariedad Hormonal: HRPC Inexacto: Cáncer de Próstata todavía responde a bajos niveles andrógenos (hormonas) Resistente a Castración: CRPC Término Favorecido pero puede ser peyorativo, negativo Resistencia Endocrina u Hormonal: ERPC Propuesto como políticamente Correcto Historia Natural del Cáncer de Próstata Under UROLOGIST care Androgen deprivation Under ONCOLOGIST care First-line therapy Therapies after LHRH agonists and antiandrogens Local therapy Death Salvage therapy Burden of disease Under the care of Symptomatic ONCOLOGIST Asymptomatic Nonmetastatic Castrate sensitive Metastatic Castrate resistant Higano C, Figg WD, Drug management of prostate cancer; 2010. Historia Natural del Cáncer de Próstata • A 20 años, el riesgo de mortalidad para pacientes 55 74 años con Gleasson 2 - 4 es Survival menor a 10 % Non–prostate cancer mortality Age at Diagnosis (Yrs) 55-59 OS 0 20 40 60 80 100 Alive, % 100 80 60 40 20 0 100 80 60 40 20 0 100 80 60 40 20 0 Gleason Score 6 Gleason Score 7 Gleason Score 8-10 0 5 10 15 20 0 5 10 15 20 0 5 10 15 20 0 5 10 15 20 Yrs Following Diagnosis Albertsen P, JAMA. 2005;293:2095-2101. 0 20 40 60 80 100 0 20 40 60 80 100 0 20 40 60 80 100 Deceased, % Other Cause Mortality 70-74 100 80 60 40 20 0 Prostate cancer mortality Prostate Cancer Mortality 60-64 65-69 Gleason Score 5 Opciones Terapéuticas para Cáncer de Próstata Sipuleucel-T*[8] LHRH Zoledronic Acid[4] agonists*[1,2] Mitoxantrone[3] 1984-1989 1996 Cabazitaxel*[7] Denosumab[9] Abiraterone*[10] Docetaxel*[5,6] 2002 2004 .... 2010 2011 MDV3100[11] * Approved agent for PCa Reversible AR blockers[1,2] Radium-223[12] However, this rapid change has left many unanswered questions, including the optimal selection and sequence of therapy 1. 1. The Leuprolide Study Group. NEJM. 1984;311:1281-1286. 2. Crawford ED, NEJM 1989;321:419-424. 3. Tannock IF, J Clin Oncol. 1996;14:1756-1764. 4. Saad F, J Natl Cancer Inst. 2002;94:1458-1468. 5. Petrylak DP, NEJM 2004;351:15131520. 6. Tannock IF, NEJM. 2004;351:1502-1512. 7. de Bono JS, Lancet. 2010;376:1147-1154. 8. Kantoff PW, NEJM 2010;363:411-422. 9. Fizazi K, Lancet. 2011;377:813-822. 10. de Bono JS, NEJM 2011;364:1995-2005. 11. Scher HI, ASCO GU 2012. 12. Parker C, et al. ASCO GU 2012. Carcinoma de Próstata Hormono Resistente Docetaxel Uso de Docetaxel reemplaza a Mitoxantrone como tratamiento estándart SWOG 99-16: Docetaxel/estramustine mejoría en sobrevida 2 meses vs Mitoxantrone 100 100 80 80 60 40 Weekly docetaxel 20 Docetaxel q3w OS (%) OS (%) TAX-327: Docetaxel mejoría en sobrevida, dolor, PSA y calidad de vida vs Mitoxantrone] Docetaxel + estramustine (217 deaths; median: 17.5 mos) 60 40 20 Mitoxantrone P = .02 Mitoxantrone + prednisone (235 deaths; median: 15.6 mos) 0 6 9 12 15 18 21 24 27 30 33 Mos 1. Tannock IF, N Engl J Med. 2004;351:1502-1512. 2. Petrylak DP, N Engl J Med. 2004;351:1513-1520. 0 12 24 Mos 36 48 Carcinoma de Próstata Hormono Resistente Cabazitaxel Segunda Línea Stratified by ECOG PS (0, 1 vs 2) and measurable vs nonmeasurable disease Patients with mCRPC progressing on docetaxel (N = 755) • • Cabazitaxel 25 mg/m2 IV q3w + Prednisone 10 mg/day PO for 10 courses (n = 378) Mitoxantrone 12 mg/m2 IV q3w + Prednisone 10 mg/day PO for 10 courses (n = 377) Primary endpoint: OS Secondary endpoints: PFS, response rate, safety de Bono JS, Lancet. 2010;376:1147-1154. Carcinoma de Próstata Hormono Resistente Cabazitaxel Segunda Línea Patients Remaining Alive (%) 100 Median OS for MP vs CBZP: 12.7 vs 15.1 mos HR : 0.72 (95% CI: 0.61-0.84; P < .0001) 80 60 40 Censored MP CBZP 20 Combined median follow-up: 13.7 mos 0 0 Patients at Risk, n MP 377 CBZP 378 6 12 18 24 30 94 137 31 60 9 19 Mos 299 321 de Bono JS, Lancet. 2010;376:1147-1154. 195 241 Data cutoff: March 10, 2010 Inmunoterapia en Cáncer de Próstata Hormono Resistente Sipuleucel-T (IMPACT) 100 Probability of Survival (%) HR : 0.78 (95% CI: 0.61-0.98; P = .03) 80 Sipuleucel-T Placebo 60 40 20 21.7 mos 0 0 12 24 36 48 60 Mos Since Randomization Kantoff PW, N Engl J Med. 2010;363:411-422. 72 Abiraterone Mecanismo de Acción Pregnenolone Deoxycorticosterone Corticosterone Aldosterone CYP17: 17α-hydroxylase 17OH-Pregnenolone 11-deoxycortisol Cortisol x2 CYP17: C17,20-lyase DHEA x3 Androstenedione Attard G, J Clin Oncol. 2008;26:4563-4571. Testosterone < 1 ng/dL Estradiol < 80 ng/dL < 2 ng/dL Inhibidores del Eje Androgénico Androgen Biosynthesis Inhibitors (ABI): Ketoconazole Abiraterone TAK700 ABI ARI Chen Y, et al. Lancet Oncology. 2009;10:981-991 Second generation AR inhibitors (ARI): Enzalutamide (MDV3100) ARN509 COU-AA-301: Abiraterona • Abiraterone aumenta SV vs Placebo con beneficio en todos los subgrupos n HR (95% CI) 1068 0.64 (0.53-0.78) <4 659 0.64 (0.50-0.82) ≥4 536 Group Baseline ECOG 0-1 BPI 0.68 (0.53-0.85) Previous chemotherapy 1 regimen 833 0.63 (0.51-0.78) 2 regimens 362 0.74 (0.55-0.99) PSA only 363 0.59 (0.42-0.82) Radiographic 832 0.69 (0.56-0.84) 363 0.70 (0.52-0.94) Progression type Visceral disease de Bono JS, N Eng J Med. 2011;364:1995-2005. Carcinoma de Próstata Hormono Resistente COU-AA-301: Abiraterona 100 HR: 0.646 (95% CI: 0.54-0.77; P < .0001) Survival (%) 80 Abiraterone acetate Median OS: 14.8 mos (95% CI: 14.1-15.4) 60 40 Placebo Median OS: 10.9 mos (95% CI: 10.2-12.0) 20 0 0 3 6 9 12 15 18 21 Mos Median OS with 2 previous chemos: 14.0 mos AA vs 10.3 mos placebo de Bono J, N Engl J Med. 2011;364:1995-2005. Median OS with 1 previous chemo: 15.4 mos AA vs 11.5 mos placebo Carcinoma de Próstata Hormono Resistente Abiraterona en Pacientes sin Quimioterapia Previa Ryan, C.NEJM 2013;368:138-48. Carcinoma de Próstata Hormono Resistente Abiraterona en Pacientes sin Quimioterapia Previa Ryan, C.NEJM 2013;368:138-48. Carcinoma de Próstata Hormono Resistente Abiraterona en Pacientes sin Quimioterapia Previa Ryan, C.NEJM 2013;368:138-48. Carcinoma de Próstata Hormono Resistente Abiraterona en Pacientes sin Quimioterapia Previa Ryan, C.NEJM 2013;368:138-48. Carcinoma de Próstata Hormono Resistente Enzalutamida AFFIRM Randomized 2:1 Enzalutamide 160 mg PO daily (n = 800) Patients with mCRPC progressing on docetaxel (N = 1199) Placebo PO daily (n = 399) • Primary endpoint: OS • Key secondary endpoints: PSA response, soft-tissue objective response, radiographic PFS, time to PSA progression Scher HI, ASCO GU 2012. . Carcinoma de Próstata Hormono Resistente Enzalutamida AFFIRM N Engl J Med 2012;367:1187-97 Carcinoma de Próstata Hormono Resistente Enzalutamida AFFIRM N Engl J Med 2012;367:1187-97 Carcinoma de Próstata Hormono Resistente Enzalutamida AFFIRM N Engl J Med 2012;367:1187-97 Carcinoma de Próstata Hormono Resistente Enzalutamida AFFIRM SRE Free (%) HR: 0.621 (P < .0001) 100 90 80 70 Enzalutamide: 16.7 mos (95% CI: 14.6-19.1) 60 50 40 30 20 10 0 Placebo: 13.3 mos (95% CI: 5.5-NYR) 0 Pts at Risk, n Enzalutamide 800 Placebo 399 3 6 676 278 548 196 De Bono JS, ASCO 2012. Abstract 4519. 9 12 15 Time to Event (Mos) 379 128 209 68 87 33 18 21 24 19 11 2 0 0 0 Carcinoma de Próstata Hormono Resistente Enzalutamida AFFIRM HR: 0.631 (95% CI: 0.529-0.752; P < .001) 37% reduction in risk of death 100 Enzalutamide: 18.4 mos (95% CI: 17.3-NYR) 50 Placebo: 13.6 mos (95% CI: 11.3-15.8) 0 0 Enzalutamide 800 Placebo 399 Scher HI, ASCO GU 2012. 6 12 701 317 400 167 OS (Mos) 18 72 33 24 7 3 0 0 Carcinoma de Próstata Hormono Resistente Enzalutamida AFFIRM N Engl J Med 2012;367:1187-97 Carcinoma de Próstata Hormono Resistente Radium-223 ALSYMPCA Stratified by total ALP, previous docetaxel, and bisphosphonate use; randomized 2:1 Up to 6 treatments at 4-wk intervals Patients with symptomatic CRPC and ≥ 2 bone metastases with no known visceral metastases, either post-docetaxel or unfit for docetaxel (N = 921) Radium-223 50 kBq/kg + BSC Placebo (saline) + BSC Primary endpoint: OS Secondary endpoints: time to first SRE, time to total ALP progression, total ALP response, ALP normalization, time to PSA progression, safety, QoL Parker C, ASCO GU 2012. Abstract 8. Carcinoma de Próstata Hormono Resistente Radium-223 ALSYMPCA 100 HR: 0.695 (95% CI: 0.552-0.875; P = .00185) 90 80 OS (%) 70 60 Radium-223 (n = 541) Median OS: 14.0 mos 50 40 Placebo (n = 268) Median OS: 11.2 mos 30 20 10 0 0 3 6 Pts at Risk, n Parker C, 2012 ASCO GU Abstract 8. 9 12 15 Mos 18 21 24 27 Carcinoma de Próstata Hormono Resistente Radium-223 ALSYMPCA All Grades Adverse Event, n (%) Grade 3/4 Radium-223 (n = 509) Placebo (n = 253) Radium-223 (n = 509) Placebo (n = 253) Hematologic •Anemia •Neutropenia •Thrombocytopenia 136 (27) 20 (4) 42 (8) 69 (27) 2 (1) 14 (6) 54 (11) 9 (2) 22 (4) 29 (12) 2 (1) 4 (2) Nonhematologic •Bone pain •Diarrhea •Nausea •Vomiting •Constipation 217 (43) 112 (22) 174 (34) 88 (17) 89 (18) 147 (58) 34 (13) 80 (32) 32 (13) 46 (18) 89 (18) 6 (1) 8 (2) 10 (2) 6 (1) 59 (23) 3 (1) 4 (2) 6 (2) 2 (1) Parker C, 2012 ASCO GU Abstract 8. Interacción entre MET y VEGFR en Tumores Óseos • MET es activado en metástasis óseas Stroma Angiogenesis VEGF – Células Tumorales expresan MET Proliferation differentiation survival HGF Osteoblast VEGF HGF HGF VEGF NP-1 – Activación del MET por HGF en forma autocrina y paracrina – Osteoblastos y Osteoclastos expresan MET y VEGFRs Zhang S, et al. Mol Cancer. 2010;9:9. MET HGF Migration proliferation survival Osteoclast VEGF Migration proliferation survival Tumor Cell Carcinoma de Próstata Hormono Resistente Rol de MET Androgen Deprivation Activates MET Signaling Stromal HGF AR MET Androgen deprivation HGF AR X MET (autocrine + paracrine) Activated MET Is Highly Expressed in Bone Metastases Zhang S, et al. Mol Cancer. 2010;9:9. Carcinoma de Próstata Hormono Resistente Cabozantinib vs Placebo Baseline Bone Scan Evaluable (N = 108) Wk 12 Complete resolution 21 (19) Partial resolution 61 (56) Stable 23 (21) Docetaxel pretreated Hussain M, ASCO 2011. Abstract 4516. % Best Change From Baseline Progressive disease 100 80 60 20 0 -20 -40 -60 -80 -100 n (%) Pts With Baseline t-ALP Levels ≥ 2 x ULN and ≥ 12 Wks of Follow-up (N = 28) 100 80 60 40 20 0 -20 -40 -60 -80 -100 3 (3) Bisphosphonate treated Bisphosphonate naive Samples From Wk 6 and 12 (N = 118) n (%) Bone metastases and bone pain at baseline (n = 83): pain improvement at Wk 6 or 12 56 (67) Narcotics for bone pain at baseline (n = 67): pain improvement at Wk 6 or 12 47 (70) Evaluable for narcotics change (n = 55): decrease or discontinuation of narcotics 7/27 (26%) patients discontinued narcotics entirely Hussain M, ASCO 2011. Abstract 4516. 31 (56) 20 0 -20 Nonrandomized Expansion Trial Prospective: Pts With Average Worst Pain ≥ 4 at Baseline ** * Improved Randomized Discontinuation Trial; Post Hoc Investigator Survey % Change in Average Worst Pain From Baseline Cabozantinib Dolor Óseo y Uso de Narcóticos -40 -60 -80 -100 Previous docetaxel Previous docetaxel + abiraterone and/or cabazitaxel *Previous radionuclide therapy Median best pain reduction from baseline: 46% Cabozantinib Estudios Fase III Randomizados Patients with bone-metastatic CRPC, moderate to severe bone pain, and previous treatment with docetaxel, abiraterone, or enzalutamide (Planned N = 246) Patients with bone-metastatic CRPC, and previous treatment with docetaxel, abiraterone, or enzalutamide (Planned N = 246) Cabozantinib 60 mg QD + Mitoxantrone Placebo Primary endpoint: durable pain response at Wk 12 Mitoxantrone/Prednisone + Cabozantinib Placebo Cabozantinib 60 mg QD + Placebo Secondary endpoints: bone scan response by IRF, OS OS Endpoint Trial[2] Primary endpoint: OS Secondary endpoints: bone scan response by IRF Prednisone 5 mg BID + Placebo 1. ClinicalTrials.gov. NCT01522443. 2. ClinicalTrials.gov. NCT01605227. Pain Endpoint Trial[1] Dasatinib Inhibición del Src • Src permanece sobreexpresado en células tumorales de Cáncer de Próstata • La función normal del Osteoclasto depende de quinasa Src • La inhibición del Src inhibe: – Proliferación de células tumorales – Proliferación de Osteoclastos – Actividad y Osteólisis de Osteoclastos Maximum PSA Change From Baseline (%) 200 Tumor Size (by RECIST) PSA 150 100 50 0 -50 -100 -150 Yu EY, Clin Cancer Res. 2009;15:7421-7428. Maximum uNTx Change From Baseline (%) 50 40 30 20 10 0 -10 -20 -30 -40 -50 160 140 120 100 80 60 40 20 0 -20 -40 -60 -80 -100 100 Maximum BAP Change From Baseline (%) Maximum Tumor Size Change From Baseline (%) Carcinoma de Próstata Hormono Resistente Dasatinib: Estudios Fase II – No Qtp previa Urine N-Telopeptide Bisphosphonate No bisphosphonate Bone Alkaline Phosphatase 80 60 40 20 0 -20 -40 -60 -80 Bisphosphonate No bisphosphonate Carcinoma de Próstata Hormono Resistente Dasatinib: Estudio Fase III Patients with metastatic CRPC and evidence of progression (Planned N = 1500) Docetaxel + Prednisone + Placebo daily Docetaxel + Prednisone + Dasatinib 100 mg/day PO Primary endpoint: OS Secondary endpoints: ∆ uNTx, time to first SRE, ∆ pain intensity, time to PSA progression, tumor response rate, PFS, safety/tolerability ClinicalTrials.gov. NCT00744497 Carcinoma de Próstata Hormono Resistente Estrategias Dirigidas a Enfermedad Ósea • Prevención de fractura en Estadios Tempranos Bifosfonatos y Denosumab cada 6 meses • Retardar la aparición de Metástasis Óseas Denosumab aumenta SLE (no uso estándart) • Tratamiento de Metástasis Óseas en Carcinoma de Próstata Hormono Resistente • Tratamiento de Metástasis Óseas en Carcinoma de Próstata Hormono Sensible Denosumab ? • Nuevos Agentes Carcinoma de Próstata Hormono Resistente Eventos Óseos: Consecuencias Clínicas Eventos Óseos Consecuencias Clínicas • • • • • • • • • • Fracturas Patológicas Compresión Medular Uso de Radioterapia Cirugía de Metásis Óseas Hipercalcemia Cambio en tratamiento Sistémico Dolor Óseo Uso de Analgésicos Calidad de Vida Menor Sobrevida Carcinoma de Próstata Hormono Resistente Parmidronato Eligibility Criteria Prostate cancer with confirmed skeletal metastases Bone pain secondary to bone metastases No previous bisphosphonate R A N D O M I Z E D SRE (Study Wk 27), n (%) Pamidronate 90 mg q3w x 9 (n = 169) Placebo q3w x 9 (n = 181) Pamidronate Placebo Any SRE 42 (25) 46 (25) Radiation to bone (pain relief) 25 (15) 29 (16) Vertebral fracture 11 (7) 10 (6) Spinal cord compression 5 (3) 3 (2) Surgery to bone 5 (3) 6 (3) Small EJ, J Clin Oncol. 2003;21:4277-4284. Carcinoma de Próstata Hormono Resistente Ácido Zoledrónico Eligibility Criteria Patients with prostate cancer Castration resistant Bone metastases (N = 643) • • • R A N D O M I Z E D Zoledronic acid 4 mg q3w (n = 214) Zoledronic acid 4 mg q3w (initially 8 mg) (n = 221) Placebo q3w (n = 208) Patients in 8-mg arm reduced to 4 mg because of renal toxicity Primary outcome: proportion of patients having ≥ 1 SRE Secondary outcomes: time to first on-study SRE, proportion of patients with SREs, and time to disease progression Saad F, J Natl Cancer Inst. 2002;94:1458-1468. Carcinoma de Próstata Hormono Resistente Ácido Zoledrónico Eventos Óseos Percent Without Event 100 80 60 40 20 Median, Days P Value 488 321 .009 ZOL 4 mg Placebo 0 0 120 240 Days 360 480 • SREs: ZOL 4 mg 38%; placebo 49% (P = .028) 11% absolute risk reduction in ≥ 1 SRE • Pain/analgesia scores increased less with ZOL • No improvement in tumor progression, QoL, OS 600 Saad F, ASCO 2003. Abstract 1523. Saad F, et al. J Natl Cancer Inst. 2004;96:879-882. 720 Carcinoma de Próstata Hormono Resistente Denosumab vs Ácido Zoledrónico Patients with CRPC and bone metastases, and no current or past IV bisphosphonate treatment (N = 1901) Denosumab 120 mg SC + Placebo IV* q4w (n = 950) Zoledronic acid 4 mg IV* + Placebo SC q4w (n = 951) Calcium and vitamin D supplemented in both treatment groups Primary endpoint: time to first on-study SRE (fracture, radiation or surgery to bone, spinal cord compression) *Per protocol and zoledronic acid label, IV product dose adjusted for baseline creatinine clearance and subsequent dose intervals determined by serum creatinine. No SC dose adjustments made due to increased serum creatinine. Fizazi K, Lancet. 2011;377:813-822. Proportion of Subjects Without SRE Denosumab vs Ácido Zoledrónico Tiempo al Primer Evento Óseo 1.00 HR: 0.82 (95% CI: 0.71-0.95; P = .0002, noninferiority; P = .008, superiority) 18% Risk reduction 0.75 0.50 KM Estimate of Median Mos 20.7 Denosumab 17.1 Zoledronic acid 0.25 0 0 3 Patients at Risk, n Fizazi K, Lancet. 2011;377:813-822. 6 9 12 15 Study Mo 18 21 24 27 Denosumab vs Ácido Zoledrónico Efectos Adversos Subject Incidence, n (%) Zoledronic Acid (n = 945) Denosumab (n = 943) Infectious adverse events 375 (39.7) 402 (42.6) Infectious serious adverse events 108 (11.4) 130 (13.8) Acute-phase reactions (first 3 days) 168 (17.8) 79 (8.4) Renal adverse events* 153 (16.2) 139 (14.7) 12 (1.3) 22 (2.3) • Yr 1 5 (0.5) 10 (1.1) • Yr 2 8 (0.8) 22 (2.3) Hypocalcemia 55 (5.8) 121 (12.8) New primary malignancy 10 (1.1) 18 (1.9) Cumulative rate of ONJ† Fizazi K, Lancet. 2011;377:813-822. Carcinoma de Próstata Hormono Resistente Algoritmo Terapéutico Maintain castration serum levels of testosterone and use denosumab or zoledronic acid with vitamin D and calcium if bone metastases are present No Symptomatic Visceral disease Sipuleucel-T Secondary hormone therapy • Antiandrogen • Antiandrogen withdrawal • Ketoconazole or abiraterone acetate (level 2B) • Steroids • DES or other estrogen Clinical trial Mottet N, Eur Urol. 2011;59:572-583. NCCN. Clinical practice guidelines in oncology: prostate cancer. v.1.2012. Yap TA, et al. Nat Rev Clin Oncol. 2011; 8:597-610. Yes Docetaxel Mitoxantrone Abiraterone acetate Palliative radiotherapy or radionuclide (radium-223 ?) for symptomatic bone metastases Clinical trial Abiraterone acetate Cabazitaxel Salvage chemotherapy Docetaxel rechallenge Mitoxantrone Secondary hormone therapy Sipuleucel-T Enzalutamide Clinical trial Gracias por su Atención