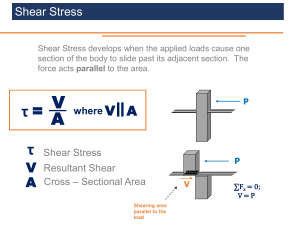
Rheology of Plastisol Formulations for Coating Applications Yamina Abdesselam,1,2 Jean-François Agassant,1 Romain Castellani,1 Rudy Valette,1 Yves Demay,1,3 Diego Gourdin,2 Richard Peres2 1 CEMEF - Center for Material Forming, CNRS UMR 7635, MINES ParisTech, PSL - Research University, Sophia Antipolis 06904, France 2 Z.A Salzbaach, Tarkett GDL, Wiltz L-9559, Luxembourg 3 Laboratoire J.A. Dieudonne, UMR CNRS 6621, Universite de Nice Sophia Antipolis, Nice 06108, France A plastisol is a suspension of PVC particles and mineral fillers in a liquid phase composed of plasticizer and adjuvants. Plastisol formulations are commonly used in coating processes for flooring application. In the knife-overroll process, they are subjected to a wide range of shear rates (0–105 s21). They are adjusted in order to fulfil the target end-use properties but their processability depends on their rheology. Plastisol based on three PVC resins with or without mineral filler have been investigated using a Couette device and a capillary rheometer. Results show a high impact of PVC particle content, particle sizes and distribution on rheology: a polydisperse formulation displays a shear-thinning behavior in the whole shear rates range and exhibits yield stress; a monodisperse formulation shows a shear thinning behavior at low shear rate, followed by a Newtonian plateau, then a more or less pronounced dilatancy peak depending on plasticizer rate and finally another shear-thinning behavior; a bidisperse resin stands in between. Filler content also impacts the rheology: shear thickening effects at intermediate shear rates decrease or even disappear; however, the viscosity increase is important for low shear rates and depends on the filler particle size and particle size distribution. POLYM. C 2016 Society of Plastics Engineers ENG. SCI., 00:000–000, 2016. V INTRODUCTION A plastisol is a suspension of PVC particles in a liquid continuous phase composed of plasticizer, adjuvants such as stabilizers, pigments, blowing agents, and sometimes, mineral fillers. Plastisol formulations are mostly used for flooring applications, which are made using different spreading processes. In the so called “knife-over-roll process” plastisol is coated at ambient temperature on a substrate between a supporting cylinder rotating at high velocity (up to 20 m/min) and a knife. A very narrow gap is adjusted between the knife and the cylinder (between 100 and 200 mm) to achieve the targeted plastisol deposited thickness (Fig. 1a). Figure 1b shows a typical knife geometry. The flooring gelation is then achieved at high temperature (140– 2008C) in an oven downstream the knife-over-roll coater [1]. When coated, the plastisol formulation is subjected to a wide shear rates range (up to 105s21) and a too high viscosity at low shear rate makes its introduction between the knife and the rotating cylinder difficult. At high shear rates, jamming effects Additional Supporting Information may be found in the online version of this article. Correspondence to: J.-F. Agassant; e-mail:jean-francois.agassant@ mines-paristech.fr DOI 10.1002/pen.24475 Published online in Wiley Online Library (wileyonlinelibrary.com). C 2016 Society of Plastics Engineers V POLYMER ENGINEERING AND SCIENCE—2016 may develop flow instabilities at knife exit. In addition, a too large separating force exerted on the knife will induce its elastic deformation, leading to a more important deposited thickness along the center line of the coating machine than at the periphery. Finally, in the industry, the trend with new formulations development is now to reduce the plasticizer content, to optimize the PVC particle size distribution and to replace an increasing proportion of PVC resin by mineral fillers, thus inducing drastic modifications of the plastisol rheology and some processing problems. The complex composition of plastisol formulations, the interaction between PVC resins and plasticizer, the PVC and filler particles size and particles size distribution, make plastisols a unique kind of suspensions. Even though typical dense suspensions rheological behavior is expected, only a few papers have been devoted to plastisol rheology at room temperature. We will discuss their behavior compared to the abundant literature on suspensions rheology. First, many authors have addressed how spherical monodisperse particles behave and influence the flowing properties of a suspension, from dilute to more concentrated systems [2–6]. A rise in viscosity is expected, as particles are added, which then diverges when a maximal volume fraction is reached, that is, packed state prevent particles to flow freely. Additionally, at low shear rates, a shear-thinning behavior is observed as the sheared suspension microstructure changes with the flow: “ordered” layers of particles are formed when Brownian effects are no more dominant, lowering the viscosity when increasing the shear rate. Accordingly, Marcilla et al. [7] showed on plastisol formulations the existence of a Newtonian plateau at very low shear rates, followed by a shear thinning behavior and a second Newtonian plateau at intermediate shear rates (around 100 s21). They pointed out that the polydispersity of PVC resin particles has an influence on the shear thinning behavior. Indeed, it is well known that adding particles of different sizes to a suspension greatly affects its rheology [8]. First, maximum volume fraction is modified, and the drastic increase in viscosity with the solid volume fraction observed for the monodisperse case is postponed to higher particle concentrations [2, 8]. Using broad particle size distributions has the same effect on rheology [9]. Size distribution impacts also the behavior of particles suspensions at high shear rates. When volume fractions are high and distributions are narrow, dilatancy, and shear-thickening effects are observed [10–18]. Particle clusters that block the flow are formed, leading to instabilities. Viscosity dramatically increases, as well as normal forces, especially in confined geometries. Nakajima et al. [19] measured such dilatancy peak on plastisols at intermediate shear rates. They also mentioned the FIG. 1. (a) Knife over-roll process and (b) typical knife geometry. presence of a second shear thinning behavior at higher shear rates. The same kind of behavior was observed by Barroso et al. [20] at low to intermediate shear rates. They used a capillary rheometer to investigate the rheological behavior up to 5000 s21. Both authors showed that the viscosity was decreasing when introducing large PVC particles (with the same particle volume fraction). This is, again, in accordance with the literature of suspensions; adding particles of different sizes usually lessens or prevents dilatancy effects [21, 22]. However, the behavior of the suspensions when the shear rate continues to increase is still open to debate: the high shear rate shear thinning behavior may be related to the clusters disintegration induced by the high stresses or by a change of the aggregates shape [16, 23]. The capacity of the particles to deform when subjected to high stresses is indeed another parameter that will affect the flow [2, 24, 25] and can be expected for plastisols as PVC particles are dispersed in a good solvent. In this article, we investigate the shear rheology of plastisol formulations of increasing complexity in the wide shear rate range encountered in the knife-over-roll process. This requires using two complementary rheometers: a Couette rheometer and a capillary rheometer. We start with the simplest formulations, which consist of a plasticizer and three different PVC, each with different particle sizes and particle size distributions, but with the same global particle content. No additive (surfactant for example) has been added to these formulations. We then vary for each formulation the PVC particle content till packing. Then we select one of these formulations and we substitute a constant fraction of calcium carbonate particles to the PVC, but keeping constant the global particle content. Finally, we test the FIG. 2. PVC resins SEM pictures. [Color figure can be viewed at wileyonlinelibrary.com] 2 POLYMER ENGINEERING AND SCIENCE—2016 DOI 10.1002/pen FIG. 3. PVC resins particle and agglomerates size distribution. influence of the calcium carbonate addition, using different particle size, but again with the same total particle content and the same proportion between PVC particles and calcium carbonate particles. The results will be compared to those of “traditional” suspensions. MATERIALS AND METHODS Plastisol Formulations Model formulations composed of PVC particles and plasticizer, with or without CaCO3 filler, have been investigated. Three PVC from Vinnolit company are selected, with different particle size distributions. Figure 2 shows SEM pictures of the three PVC used in those plastisols. Two different scales may be observed. On the first line, agglomerates with characterized sizes between 10 and 50 mm are shown. The second line points out very different elementary particles with more or less spherical geometries for the three resins: PVC 1 (reference EP 7091) has been produced by emulsion polymerization. It presents a lot of submicronics particles and a maximum particle diameter around 1 mm. PVC 2 (reference MP 7151) is produced by micro-suspension polymerization. It shows two characteristic particle diameters (0.1 and 1 mm). And PVC 3 (reference P70), also obtained by micro-suspension, is rather monomodal with particles diameter around 1 mm and a small amount of smaller ones. The shape of these particles is less spherical than the other two PVCs. The volume particle size distributions are detailed in Fig. 3. These distributions are quite different for the three PVC, but the characteristic sizes correspond rather to the agglomerates than to the elementary particles. These data, provided by PVC suppliers, were obtained by dispersing PVC in water. Therefore, it might not exactly represent the particles size distribution in plasticizer, especially when subjected to high shear rates, which may destroy the agglomerates. Particles, which diameter size is <0.5 mm, do not appear on Fig. 3: even if they are numerous as for PVC 1 and PVC 2, their contribution to the volume distribution is limited (1000 particles of diameter 0.1 mm will be equivalent to a single particle of diameter 1 mm!). PVC 1 presents an almost continuous distribution of particles from 1 to 40 mm. In what follows, it will be called “polydisperse.” PVC 2 where micrometric particles coexist with bigger ones of about 10 mm will be called “bidisperse.” PVC 3 composed of a wide DOI 10.1002/pen population of particles, which diameter around 1–2 mm, will be called “monodisperse.” Three calcium carbonate fillers of three different characteristic sizes were used, respectively called D10, D15, and D40 depending on their diameters. Filler D10 has a median diameter of 9 mm (i.e., 50% of the particles are under this size), filler D15 has a median diameter of 20 mm and D40 has the highest median diameter of 30 mm. These particle fillers have a median diameter, which is ten times higher than the elementary PVC particles and which is of the same order of magnitude than the PVC agglomerates. The shapes of these calcium carbonate particles are much more distorted than the shape of PVC elementary particles. The plasticizer used for these formulations is DINP (diisononyl phthalate), a phthalate plasticizer with a Newtonian viscosity of 0.05 Pa.s. The three first columns of Table 1 present the formulations F1, F2, and F3 of Fig. 2, each composed of one PVC in the plasticizer at a constant solid volume fraction of 53.7%. In the following, this solid volume fraction will be varied between 45% and the maximum solid volume fraction. The last three columns correspond to formulation F2 with a constant proportion of the three calcium carbonate fillers (volume fraction CaCO3/ PVC equals to 47.6%) and a global solid volume fraction (PVC 1 CaCO3) of 63.9%. In the following, we will vary the total solid volume fraction between 60 and 65%, but keeping the same proportion between calcium carbonate and PVC particles. The solid particle volume fraction has been deduced from the specific volume of plasticizer, PVC, and calcium carbonate. Preparation of the Different Formulations Plastisol formulations were prepared by adding PVC resins and CaCO3 filler to the plasticizer (DINP). The composition was then mixed at speed 1500 RPM with a Dispersmat blender for three minutes. The dissipated energy has been measured. Due to the plastisol sensitivity to temperature, this preparation protocol was chosen to enable a good dispersion and avoid a premature gelling due to temperature rise. We can mention that the mixing energy for F1 formulation was two or three orders of magnitude larger than for F2 and F3 formulations. TABLE 1. Compositions and names of the different plastisol formulations. Formulations (g): Unfilled Product F1 PVC resin 1 PVC resin 2 PVC resin 3 Filler D10 Filler D15 Filler D40 Plasticizer (DINP) Total (g) Solid volume fraction (%) 100 F2 Filled F3 100 F2D10 F2D15 F2D40 100 100 100 100 175 175 60 160 53.7 60 160 53.7 60 160 53.7 75 350 63.9 75 350 63.9 175 75 350 63.9 PVC, CaCO3 filler, and plasticizer contents are given in grams; solid volume fractions of each formulation were calculated accordingly. POLYMER ENGINEERING AND SCIENCE—2016 3 rheology of such system but some authors point out an influence for smaller gaps [26]. Capillary Rheology. For all capillary lengths, constant pressure values at plateau have been measured. Bagley plots [27] show a nice linear regression between the pressure measurements with the three capillary lengths. The viscosity as a function of the apparent shear rate is obtained (Fig. 4). The Rabinowitsch corrections, which allow obtaining the true shear rate [28], have been applied. Comparison between the Two Measurements. Couette and FIG. 4. Comparison between Couette flow tests and Capillary tests on F2 (63% volume fraction) formulation. Rheology Measurements Previous rheological studies on plastisol formulations at ambient temperature did not systematically investigate their behavior on a large shear rates range, as the ones that are encountered on industrial processes. To do so, two types of rheometer were used at room temperature. -Anton Paar MCR 301 and MCR 302, both equipped with a Couette (concentric cylinders) geometry (ref CC17 for MCR 301: 0.71 mm gap, bob height 30 mm; and ref CC27 for MCR 302: 1.13 mm gap, bob height 40 mm). This type of rheometer was used in flow mode at 258C. Shear rates range from 0.1 to 4000 s21. Depending on the formulation, the measurements performed at the highest shear rates are not relevant because they correspond to the saturation of the Couette torque measurement. -A Gottfert Rheo-Tester capillary rheometer with a 0.93 mm capillary die (silicon carbide) 3 length-to-diameter ratios (L/D: 4, 8, and 16) and a 10 MPa transducer. With this type of machine and the configuration we used, shear rates range is from 200 to 20,000 s21. A satisfying overlapping of the rheology measurements with these two kinds of rheometers is obtained. For each test, paste formulations samples, prepared following the protocol described in 2.2, were quickly remixed by hand before pouring them in the rheometer to prevent segregation of the suspension between the preparation and the rheology measurement. Influence of plastisol ageing on rheology was studied on 15 h tests; no effect was observed. capillary measurements are consistent (Fig. 4), providing a continuous rheology curve in a wide shear rate range (0.1–104 s21). Figure 4 is consistent with Nakajima et al. [19] and Barroso et al. [20] experiments: a first shear-thinning zone at low shear rates (1021210 s21) is followed by a Newtonian plateau (10– 40 s21), a shear-thickening behavior (40–600 s21) and then a second shear-thinning zone (>600 s21). This high shear rate behavior has been already observed for example by Barroso [20]. In what follows we will observe how this shape of the viscosity curve will change when modifying the PVC particle size distribution, the PVC particle content, or when substituting PVC particles by calcium carbonate particles. Influence of the PVC Resin Particle Size Distribution Figure 5 compares the Couette viscosity of the three different neat plastisol formulations at solid volume fraction 57.3%, which differ only by their particles size distributions. Due to the lower particle content, the shear rate may vary between 0.1 and 3000 s21. Very different behaviors are observed. -A sharp shear thinning behavior is observed for the polydisperse plastisol formulation F1 with a slope discontinuity above 100 s21, which could correspond to the sample rupture within the Couette gap. It is to notice that it was not possible to perform capillary measurements with this formulation because filling the capillary reservoir at such high viscosity entraps a lot of air bubbles. There is no Newtonian plateau at intermediate shear rates, nor dilatancy peak at higher shear rates. This may be explained by the high among of small particles observed Fig. 3 [22]. On the contrary, at low shear rates a broad particle size RESULTS AND DISCUSSION Protocols Validation: Study of a Model Formulation (F2 with 63% Solid Volume Fraction) Couette Rheology. Two different protocols were used for Cou- ette rheology measurements in continuous flow: (i) a continuous logarithmic increase at a rate of 5.5.1023 decade/s and (ii) shear rate steps (500 s each) to wait for flow stabilization. The imposed shear rates were set between 0.1 and 200 s21. Both continuous and step rate tests were consistent which points out that there is no thixotropic effects with these formulations. In these conditions, flow tests, which are easy and fast, were proven to be sufficient to describe plastisols steady shear behavior. Additionally, we did not find any influence of the gap size on 4 POLYMER ENGINEERING AND SCIENCE—2016 FIG. 5. Couette viscosity as a function of shear rate for F1, F2, and F3 at 57.3% particles volume fraction. DOI 10.1002/pen FIG. 6. F1 (polydisperse formulation) flow tests for different increasing PVC volume fractions (%). distribution will induce a structuration of the suspension, which will prevent the plastisol to flow, inducing a yield stress, which will be pointed out later, on Fig. 6. -The bidisperse formulation F2 shows a quasi-Newtonian behavior at a solid particle volume fraction less than in Fig. 4. -The monodisperse formulation F3 shows a slight shear thinning behavior at low shear rate followed by a shear thickening peak at higher shear rates and then by a second shear thinning behavior at the highest rates. This shape is qualitatively equivalent to the one of F2 formulation at higher particle content (63% instead of 57.3%) but the viscosity is lower. The shearthickening effect observed between 10 and 500 s21 may be explained by the creation of chains of contacts between the particles and the walls of the rheometer [10–18]. This dilatancy peak is not present when the formulation is composed of particles of different diameters (F1) due to the lubrication induced by the smallest particles that prevent the system to jam. The second shear thinning behavior at high shear rates was already observed for resin F2 but at higher solid volume fraction. This was previously described in the literature [11, 16] and a possible explanation is the destruction of the chains of contact induced by high stresses, but the relative softness of the PVC particles in such a good solvent [16] may also allow deformation and flow even when a jammed state is reached. FIG. 7. F2 (bidisperse formulation) flow tests for increasing PVC volume fractions (%). DOI 10.1002/pen FIG. 8. F3 (monodisperse formulation) flow tests for different increasing PVC volume fractions (%). Influence of the PVC Particle Volume Fraction Couette flow tests were performed on these three formulations at different solid volume fractions ranging between 45 and 66.6%. These tests show a sharp shear thinning behavior for polydisperse PVC formulation F1 whatever the plasticizer content (Fig. 6). Rheology measurements were not possible for a solid volume fraction higher than 63.5% because the high viscosity of the formulation at low shear rate prevents the Couette gap filling. On F1 formulations, a yield stress is also observed (highlighted on Fig. 6 by the 21 slope at small shear rates). It varies between 150 Pa for a solid volume fraction of 45% to 2000 Pa for a solid volume fraction of 63.5%. This behavior has been previously pointed out by Zhou et al. [29] For bidisperse formulation F2 (Fig. 7) a quasi-Newtonian behavior is observed at low solid volume fraction. For high solid volume fraction, a slight shear thinning behavior is observed FIG. 9. Influence of substituting filler particles to PVC particles on the viscosity of PVC F2 formulations at two different total solid volume fractions. POLYMER ENGINEERING AND SCIENCE—2016 5 diameter 9 mm). Figure 9 shows the Couette rheology measurements for two different total solid volume fractions. Introducing the finest CaCO3 particles leads to a significant increase of the viscosity at low shear rates (a factor 3 at 1 s21) and a marked shear thinning effect. At higher shear rates, the dilatancy peak disappears as shear thickening is spread out over a larger range of shear rates. For these solid particle volume fractions, the viscosity between 100 and 1000 s21 is equivalent to the viscosity of the F2 formulation. FIG. 10. Viscosity measurements for different bidisperse based formulations: the total solid volume fraction is 64%. F2 is the neat PVC formulation; F2D10, F2D15, F2D40 correspond to the substitution of different CaCO3 filler particle sizes with a constant proportion of 47.6%. Couette (plain symbols) and capillary (empty symbols). at low shear rates, followed by a Newtonian plateau at intermediate shear rates, a slight viscosity increase at higher shear rates and finally a viscosity decrease above 500 s21. Monodisperse formulation F3 (Fig. 8) shows, whatever the PVC particles volume fraction, a slight shear thinning behavior at low shear rate followed by a Newtonian plateau at intermediate shear rates, then a marked dilatancy peak and finally a second shear thinning zone at higher shear rates. As soon as the particle content exceeds 56%, a sudden jump of the rheometers torque (and so of the viscosity) may be observed (two orders of magnitude of viscosity jump). The shear rate at which this viscosity jump is observed decreases when the particle content increases (40 s21 at 58% PVC particle content and 2 s21 at 66.6%) and the viscosity jump increases too. This discontinuous shear thickening effect has been observed for many suspension systems [10–18]. Its origin is subjected to many speculations [30]. For the bidisperse F2 and monodisperse F3 formulations, the minimum viscosity at the Newtonian plateau has been drawn as a function of the PVC particle solid volume fraction (Supporting Information, Figure A). We apply then the Quemada Eq. 1 without parameter fitting [6]: g5g0 / 12 /max 22 (1) g0 is the plasticizer viscosity (here 0.05 Pa.s), / the solid volume fraction and /max the maximum solid volume fraction, which has been determined experimentally by mixing increasing amount of PVC resin in plasticizer until the mixing is no longer possible. The /max value for the monodisperse formulation F3 is 67.3%, which is a little higher than the theoretical value of 64%; this may correspond to the small amount of smaller particles observed on Fig. 2. The bidisperse formulation F2 presents logically a higher /max value (69.9%). This fitting to the Quemada equation was performed without any adjustable parameters and it gives noticeably satisfactory results. Influence of Calcium Carbonate Particles Substituting PVC Particles by Calcium Carbonate Particles at the Same Total Volume Fraction. A fraction of PVC particles (47.6%) has been replaced by CaCO3 D10 particles (median 6 POLYMER ENGINEERING AND SCIENCE—2016 Influence of the Filler Particle Size. CaCO3 particles of different sizes (D10, 9 mm; D15, 20 mm; D40, 30 mm) have been now substituted in the same proportion (47.6%) to the PVC particles of formulation F2 at the same global solid volume fraction (64%). Figure 10 shows the resulting viscosity curves using a Couette and a capillary rheometer. Couette and capillary curves are consistent as in Fig. 4. It is interesting to notice that there is some overlapping between shear rate ranges in Couette and capillary measurements, which was not the case in previous studies on such suspensions [20]. In addition the Couette and capillary measurements are consistent for both unfilled F2 formulation and the formulation with the smallest calcium carbonate particles (F2D10) The agreement is less fair for the biggest calcium carbonate particles but the discrepancy remains limited (between 1 and 2 Pa.s). Several remarkable phenomena are observed: -Substituting the PVC particles (9 mm) with the finest CaCO3 particles (9 mm) results, as previously observed, in a sharp increase of the viscosity at low shear rates; introducing the coarser particles (20 and 30 mm) leads on the contrary to a viscosity decrease in the whole shear rate range, as more pronounced as the size of the filler particles increases. The medium size of the coarser calcium carbonate particles is higher than the biggest F2 formulation PVC particles (Fig. 3); these particles will represent a distinct second population of solid particles and this will induce a viscosity decrease. -The dilatancy peak disappears for all the formulations. -At high shear rates, and especially in the second shear thinning zone, the viscosity of the formulations with filler content is lower than the viscosity of the pure PVC formulation (a factor 3 for the formulation with the coarser CaCO3 particles). Influence of the (PVC and Filler Particle) Global Content. Several F2D10 formulations (PVC F2 1 CaCO3 9 mm) have been tested with an increasing solid particle volume fraction but with the same proportion of PVC and filler particles (Supporting Information, Figure B). When the solid volume fraction increases, the viscosity curves are shifted. A slight shear thickening effect may be observed at high shear rates for the higher particle content. APPLICATION TO THE KNIFE-OVER-ROLL COATING PROCESS The plastisol flow between the knife and the roll was computed using the lubrication theory. It is a classical and efficient way to determine the pressure profile of a flow in a small gap contraction. If the liquid behaves as a Newtonian liquid, the key point is the use of the formula expressing the flow rate of a plane Poiseuille flow in the small gap between the knife and the DOI 10.1002/pen roll combined with mass conservation. If the liquid does not behave as a Newtonian liquid, which is the case for all the investigated formulations (Fig. 4) this flow rate is no more obtained by using a closed formula but it can be computed numerically as a nonlinear function of the pressure drop by using a smoothed relationship between shear stress and shear strain deduced from Fig. 4. It is then possible to compute the pressure profile between the knife and the roll combining technical, but simple in the principle, numerical strategies to solve non-linear equations. Supporting Information data figure C thus illustrates the wide range of shear rates encountered in the knife-over-roll process. CONCLUSIONS The shear viscosity of plastisol formulations is very different from the viscosity of other thermoplastic polymers that can be used for coating. Plastisol formulations behave as suspensions and some phenomena may be explained in the light of the abundant literature on this field: influence of particle size and particle size distribution, jamming effects at high shear rates. Nevertheless the interactions between the PVC particles and the plasticizer at ambient temperature, the coexistence of smooth spherical PVC particles and rough fractal calcium carbonate particles, makes this suspension a more complex system than those encountered in the literature and in other industrial applications. It has been seen that modifying even in small proportions the particle size distribution, the plasticizer content and substituting PVC particles by mineral filler particles, has a drastic influence on the rheological behavior. For processing purposes, as illustrated in supplementary data figure C, one needs to master the viscosity in the whole shear rate range. A polydisperse resin may induce a high viscosity at the low shear rates encountered in the inlet zone of the knife over roll process and this may prevent the plastisol to enter in the gap between knife and roll. The same problem may occur when using a bidisperse formulation with small diameter calcium carbonate particles. Otherwise, a monodisperse resin may induce jamming effects, which could block the plastisol flow at high shear rates in the downstream zone of the process. Introducing coarse calcium carbonate particles in a bimodal formulation will simultaneously limit the viscosity at low shear rates and decrease or even suppress the dilatancy peak at high shear rates. Nevertheless, some defects may appear on the final flooring product depending on the knife geometry, the gap between the knife and the roll and on the plastisol formulation. The shear rheology is not the unique factor for the development of these defects. DOI 10.1002/pen REFERENCES 1. Y. Georget, Tech. Ing. Plast. Compos., A3665 (1996). 2. D.B. Genovese, Adv. Colloid Interface Sci., 171–172, 1 (2012). 3. I.M. Krieger and T.J. Dougherty, Trans. Soc. Rheol., 3, 137 (1959). 4. W.B. Russel and P.R. Sperry, Prog. Org. Coat., 23, 305 (1994). 5. H.J.H. Brouwers, Phys. Rev. E, 81, 051402 (2010). 6. D. Quemada, Rheol. Acta, 17, 632 (1978). 7. A. Marcilla, J.C. Garcia, and M. Beltran, Eur. Polym. J., 33, 753 (1997). 8. R.J. Farris, Trans. Soc. Rheol., 12, 281 (1968). 9. P.F. Luckham and M.A. Ukeje, J. Colloid Interface Sci., 220, 347 (1999). 10. M.E. Cates, M.D. Haw, and C.B. Holmes, J. Phys. Condens. Matter, 17, S2517 (2005). 11. O. Hess and S. Hess, Phys. A, 207, 517 (1994). 12. Y.S. Lee, and N.J. Wagner, Rheol. Acta., 42, 199 (2003). 13. R.L. Hoffman, J. Rheol., 42, 111 (1998). 14. E. Brown, N.A. Forman, C.S. Orellana, H. Zhang, B.W. Maynor, D.E. Betts, J.M. DeSimone, and H.M. Jaeger, Nat. Mater., 220 (2010). 15. J. Bender, and N.J. Wagner, J. Rheol., 40, 899 (1996). 16. N.J. Wagner, and J.F. Brady, Phys. Today, 62, 27 (2009). 17. E. Brown, and H.M. Jaeger, Rep. Prog. Phys., 77, 046602 (2014). 18. B.J. Maranzano, and N.J. Wagner, J. Rheol., 45, 1205 (2001). 19. N. Nakajima, and E.R. Harrell, J. Appl. Polym. Sci., 115, 3605 (2010). 20. E.G. Barroso, F.M. Duarte, M. Couto, and J.M. Maia, Polym. Test., 29, 419 (2010). 21. C.E. Chaffey, Colloid Polym. Sci., 255, 691 (1977). 22. W.H. Boersma, J. Laven, and H.N. Stein, AIChE J., 36, 321 (1990). 23. A. Fall, F. Bertrand, D. Hautemayou, C. Mezière, P. Moucheront, A. Lema^ıtre, and G. Ovarlez, Phys. Rev. Lett., 114, 098301 (2015). 24. C. Servais, R. Jones, and I. Roberts, J. Food Eng., 51, 201 (2002). 25. C. Pozrikidis, Eur. J. Mech.–B/Fluids, 24, 125 (2005). 26. A. Fall, N. Huang, F. Bertrand, G. Ovarlez, and D. Bonn, Phys. Rev. Lett., 100, 018301 (2008). 27. E.B. Bagley, J. Appl. Phys., 28, 193 (1957). 28. B. Rabinowitsch, Z. Phys. Chem., 145, 1 (1929). 29. Z. Zhou, M.J. Solomon, P.J. Scales, and D.V. Boger, J. Rheol., 43, 651 (1999). 30. M. Wyart and M.E. Cates, Phys. Rev. Lett., 112, 098302 (2014). POLYMER ENGINEERING AND SCIENCE—2016 7