Thallium Reduction & Reducing Sugar Analysis - Chemistry Article
Anuncio
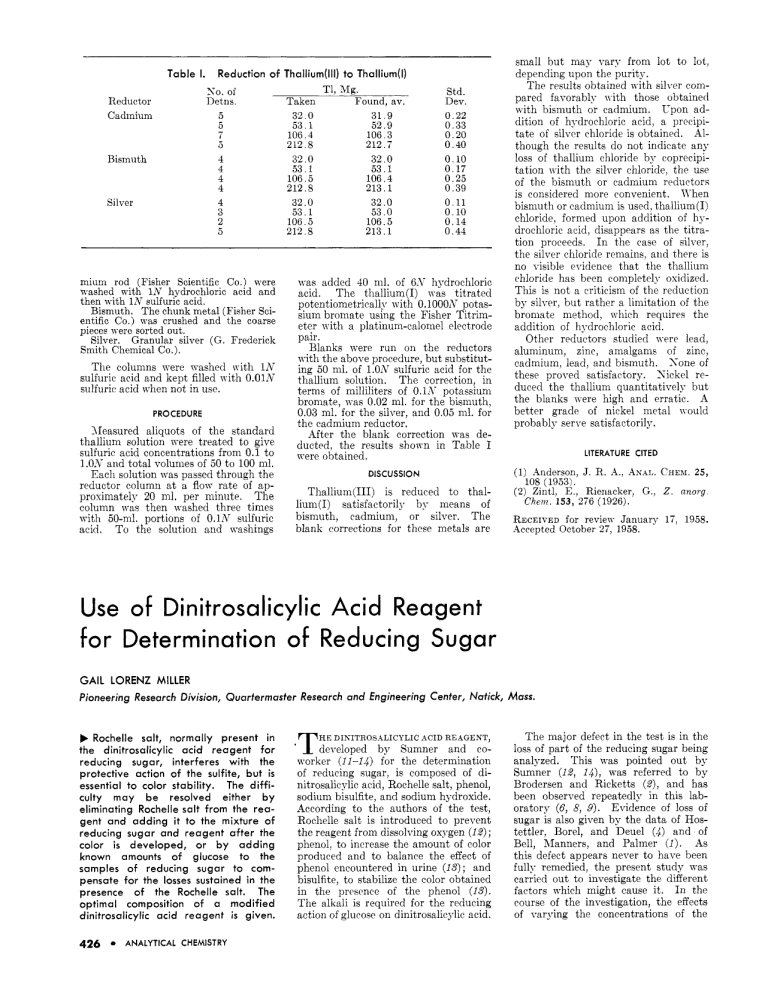
Table I. Reduction of Thallium(lll) to Thallium(1) K O . of Detns. Reductor Cadmium 5 5 7 a Bismuth 4 4 4 4 4 3 2 5 Silver mium rod (Fisher Scientific Co.) were washed with lil' hydrochloric acid and then with 1N sulfuric acid. Bismuth. The chunk metal (Fisher scientific Co.) was crushed and the coarse pieces were sorted out. Silver. Granular silver (G. Frederick Smith Chemical Co.). The columns were washed nith 1K sulfuric acid and kept filled n i t h 0.01N sulfuric acid when not in use. PROCEDURE Measured aliquots of the standard thallium solution were treated to give sulfuric acid concentrations from 0.1 to l.O*V and total volumes of 50 to 100 ml. Each solution was uassed through the \Tit11 jo-ml. portions of 0.1N sulfuric acid. To the solution and washings T1, Mg. Found, av. 31.9 5'2.9 106,3 212.7 32.0 53.1 106.4 213.1 32.0 53.0 106.5 106.5 212.8 213.1 Taken 32.0 53.1 106.4 212.8 32.0 53.1 106.5 212.8 32.0 53.1 Std. Dev. 0.22 0.33 0.20 0.40 0.10 0.17 0.25 0.39 0.11 0.10 0.14 0.44 was added 40 nil. of 6-Y hydrochloric acid. The thallium(1) lyas titrated potentiometrically with 0.1000A7 potassium bromate using the Fisher Titrimeter with a platinum-calomel electrode pair* Blanks were run on the reductors with the above procedure, but substituting 50 nil, of l,oLvsulfuric acid for the thallium solution. The correction, in terms of milliliters of 0.1s potassium bromate, was 0.02 ml. for the bismuth, 0.03 ml. for the silver, and 0.05 ml. for the cadmium reductor. After the blank correction was deducted, the results shown in Table I were obtained. small but may vary from lot to lot, depending upon the purity. The results obtained with silver compared favorably n ith those obtained a i t h bismuth or cadmium. Upon addition of hydrochloric acid, a precipitate of silver chloride is obtained. Although the results do not indicate any loss of thallium chloride by coprecipitation with the silver chloride, the use of the bismuth or cadmium reductors is considered more convenient. K h e n bismuth or cadmium is used, thallium(1) chloride, formed upon addition of hydrochloric acid, disappears as the titration proceeds. I n the ease of silver, the silver chloride remains, and there is no visible rridence that the thallium chloride has been completely oxidized. This is not a criticism of the reduction by silver, but rather a limitation of the bromate method, which requires the addition of hydrochloric acid. Other reductors studied were lead, aluminum, zinc, amalgams of zinc, cadmium, lead, and bismuth. S o n e of these proved satisfactory. Sickel reduced the thallium quantitatively but the blanks nere high and erratic. A better grade of nickel metal nould probably serve satisfactorily. LITERATURE CITED DISCUSSION (1) Anderson, J. R. A,, rlx.4~.CHEBI.2 5 , 108 (1953). (2) Zintl, E., Rienacker, G., 2. anorg Chem. 153, 276 (1926). bismuth, cadmium, or silver. The blank corrections for these metals are RECEIVEDfor revieTY January 17, 1958. Accepted October 27, 1958. Use of DinitrosaIicyIic Acid Reagent for Determination of Reducing Sugar GAIL LORENZ MILLER Pioneering Research Division, Quarfermasfer Research and Engineering Center, Natick, Mass. b Rochelle salt, normally present in the dinitrosalicylic acid reagent for reducing sugar, interferes with the protective action of the sulfite, but i s essential to color stability. The difficulty may be resolved either b y eliminating Rochelle salt from the reagent and adding it to the mixture of reducing sugar and reagent after the color i s developed, or b y adding known amounts of glucose to the samples of reducing sugar to compensate for the losses sustained in the presence of the Rochelle salt. The optimal composition of a modified dinitrosalicylic acid reagent is given. 426 ANALYTICAL CHEMISTRY *T HE D I N T R O S A L I C Y L I C ACID REAGENT, developed b y Sumner and coworker (11-14 for the determination of reducing sugar, is composed of dinitrosalicylic acid, Rochelle salt, phenol, sodium bisulfite, and sodium hydroxide. According to the authors of the test, Rochelle salt is introduced to prevent the reagent from dissolving oxygen (12); phenol, to increase the amount of color produced and to balance the effect of phenol encountered in urine ( I S ) ; and bisulfite, to stabilize the color obtained in the prescnce of the phenol ( I S ) . The alkali is required for the reducing action of glucosc on dinitrosalicylic acid. The major defect in the test is in the loss of part of the reducing sugar being analyzed. This was pointed out by Sumner (12, 1 4 , was referred to by Brodersen and Ricketts (Z), and has been observed repeatedly in this laboratory (6, 8, 9). Evidence of loss of sugar is also given by the data of Hostettler, Borel, and Deuel (4) and of Bell, hlanners, and Palmer ( 1 ) . As this defect appears never to have been fully remedied, the present study was carried out to investigate the different factors which might cause it. I n the course of the investigation, the effects of varying the concentrations of the different components of the reagent also were determined. The findings which resulted have led to the development of a modified reagent and procedure. IROCH. TR€AT€D SALTS^ I 63 METHOD The color tests were made with 3-nil. aliquots of reagent added to 3-ml. aliquots of glucose solution in 14-mm. tubes. The mixtures were heated for 5 minutes in a boiling water bath and then cooled under running t a p n-ater adjusted to ambient temperature. Cooling to ambient temperature was made necessary by the effect of temperature on the absorbance of the colored reaction product (w), a n effect confirmed by the present studies. The color intensities nere measured in a Beckman llodel DL spectrophotometer a t 575 nip n ith a slit n idth of 0.06 mm. The reagent of Suniner and Sisler (14) and a modified reagent w r e used in the tests. The former contained approximately 0.63Yc dinitrodicylic acid, 18.2% Rochelle salts, 0.57, phenol, 0.5% sodium biqulfitc, and 2.14% sodium hydrouide; the modified reagent coiitained 1% dinitrosalicylic acid, 0.2% phenol, 0.057c sodium sulfite, and 1% sodium hydroxide. For certain tests the modified reagent included varying concentrations of Rochelle salt. The composition chosen for the modified reagent n-as based on the resultq of preliminary tests n hich indicated that such a reagent was optimal and ~ o u l ds e n e best as the basis of rrference for testing effects of variation 111 composition. I n the abqcnce of Rochelle salt, the color obtained n ith the modified reagent n as unstable. T o stabilize the color under these conditions, 1 ml. of a 40% solution of the salt was added to the mixture of reactants subsequrnt to the developnicnt of the color and prior to cooling. The modified reagent n as prepared by placing all solid components in a container and dissolving them simultaneously by stirring with the required volume of sodium hydroxide solution. This was much simder than other mocedures (3, 14). The modified reagent produced the same color with glucose from day to day, thus proving more stable in this respect then the reagent of Brodersen and Ricketts ( 2 ) . Modified reagent to which Rochelle salt vias added also did not change from day to day in this respect. Depending upon storage conditions, however, the modified reagent tended eventually to deteriorate from atmospheric oxidation of the sulfite present. Deteriorated reagent was rejuveiiated by the addition of fresh sulfite. The danger attendant upon oxidation of sulfite could be avoided by preparing the reagent in large batches without sulfite, the sulfite being added to aliquots just prior to the time when the reagent \vas to be used. STUDY OF VARIABLES Removal of Dissolved Oxygen. OF KITROGEN.Sumner WITHSTREAM 0 0.1 0.2 0 fbl 0.3 06 0 fc) 03 06 fdJ 03 CITRATE 0 0.3 (e/ 0.6 0 03 06 ffJ MILLIGRAMS 0 03 fgl 06 0 0.3 fhl 06 GLUCOSE Figure 1. Effect of variables on color produced with glucose and dinitrosalicylic acid reagent a. b. C. d. e. f. 9. h. Stream of nitrogen passed through mixture; Sumner’s reagent Sodium sulfite treatment piior to addition of Suniner’s reagent Rochelle salt concentration; modified reagent Sulfite concentration; modified reagent Podium hydroxide concentration; modified reagent Phenol concentration; modified reagent Dinitrosalicylic acid concentration; modified reagent Carbox) methl ]cellulose, citrate, and miitures of both present; modified reagent and Sisler (14) indicated t h a t t h e loss of glucose with t h e dinitrosalicylic acid reagent was due t o destruction b y oxidation, and based their statement on unreported results of experiments in which a stream of nitrogen was passed through t h e reactants. An attempt to confirm this observation in the present nork indicated that passing a stream of nitrogen through a mixture of Sumner‘s reagent and glucose for 2 minutes prior to the development of color largely eliminated the destruction of glucose (Figure 1, a ) . WITH STLFITE. As sulfite had previously been used successfully for removing dissolved oxygen from aqueous solutions (b), it was surprising that the sulfite present in Sumner and Sisler’s reagent failed to accomplish this purpose. T o test whether this failure of the sulfite may have been due to interfereiice by other components of the reagent, sulfite a t a level of 0.1% n-as added to glucose samples prior to mixing them with the reagent. This procedure reduced the destruction of glucose by about 707,. The rewlts, shown in Figure 1, b, thus provided strong evidence for the suspected interference by the other components of the reagent under the usual conditions of the test. INTERFEREWE OF ROCHELLESALT. Comparative tests were next carried out with the modified dinitrosalicylic acid reagent to M hich varying amounts of Rochelle salt were added. The results, s h o r n in Figure 1, c, clearly implicated Rochelle salt as the major factor involved in the interference with the removal of oxygen by sulfite, because, in the absence of the salt, the sulfite appeared able to remove the dissolved oxygen and thereby to protect the glucose. iiside from contributing to the loss of a portion of the glucose, the Rochelle salt caused an enhancement of the color due to the remaining glucose. Sulfite Concentration. T h e effect of different concentrations of sulfite in the modified reagent (Figure I , d ) indicated t h a t a maximum color intensity n a s obtained at 0.057,sulfite. I n experiments not shown, essentially t h e same results were obtained a t 0.025 and 0.1% sulfite as a t t h e 0.057, level. Low concentrations caused a lack of linearity, nhile both high and low Concentrations caused a depression in color intensity and a loss of glucose. Sodium Hydroxide Concentration. T h e effect of different concentrations of sodium hydroxide is shown in Figure 1, e . High concentrations of sodium hydroxide led t o enhanced color development, b u t a t t h e same time contributed t o a loss of glucose. T h e level of 1% sodium hydroxide appeared t o be t h e most suitable, as it produced t h e maximum color inVOL. 31, NO. 3, MARCH 1959 * 427 tensity possible without concomitant loss of glucose. Phenol Concentration. Maximum color development was approached a t a concentration of about 0.2% phenol (Figure 1, f ) . I n experiments not shown in t h e figure, the same results were obtained with 0.5q.’, phenol as at t h e 0.2% level. Low concentrations resulted in a lack of linearity. T h e intensity obtained in t h e presence of 0.2% phenol was about 5 times that obtained in the abscence of phenol. Over the range tested, the phenol had no effect on the loss of glucose. Dinitrosalicylic Acid Concentration. When t h e amounts of dinitrosalicylic acid Fyere varied, t h e color intensity approached a maximum at a concentration of 1% (Figure 1, 9). T h e dinitrosalicylic acid, like t h e phenol, had no effect on t h e loss of glucose over t h e range tested. Other Substances. T h e results of the preceding tests suggested the possibility t h a t other substances might affect t h e dinitrosalicylic acid test. For example, it was of interest t o asceitain whether, in using t h e test for the measurement of cellulase activity (8, IO), the presence of carboxymethylcellulose and citrate buffer a t p H 5 might cause interference. K i t h amounts of carboxymethylcellulose and citrate buffer corresponding to those used in the cellulase measurement, the effects shown in Figure 1, h, were produced. The carboxymethylcellulose caused an enhancement in color, whereas the citrate caused a depression. A mixture of the substances approximately neutralized the t n o opposite effects. To determine whether the effect of the citrate may have been a consequence of its buffering action, tests ere made with acetate buffer of p H 5 a t an equivalent concentration. The acetate did not, however, affect the test. FINAL M E T H O D Khen pure reducing sugar solutions are involved or when any contaminants which may be present are knorvn not to affect the color development or to cause any loss of reducing sugar, the modified reagent in the absence of Rochelle salt is used. For stabilization of the color produced under such conditions, Rochelle salt is added to the mixture immediately after the development of the color and before the mixture is cooled. The time of heating is increased to 15 minutes because the 5- 428 ANALYTICAL CHEMISTRY minute period, adequate for the original procedure, does not produce complete color development in the modified procedure. By this method linearity of data, protection of glucose, and stability of color are realized. If interfering substances occur in unknown samples, special controls are run. Such controls consist of standard reducing sugar solutions to n hich appropriate amounts of the interfering substances are nddrd. K h e n the interfering substanctis bring about a loss of reducing sugar, and particularly when the amounts of reducing sugar to be measured in unknown samples are equal to or smaller than the amount lost, known amounts of glucose are added to both the unknown samples and the standards. The procedure of adding glucose can also be applied advantageously to compensate for the loss of reducing sugar incurred nhen Rochelle salt is incorporated in the dinitrosalicylic acid reagent. For example, it is convenient in the cellulase test (IO) to introduce glucose into the carboxymethyl-cellulose-citrate substrate and to use modified dinitrosalicylic acid reagent containing 20% Rochelle salt (8). Under these conditions the separate addition of Rochelle salt to the reaction mixture after color dcrelopment is omitted. The controls for such tests consist of a blank and standard glucose solutions, each containing carboxymethylcellulose, citrate, and compensatory glucose. DISCUSSION The chemistrv of the dinitrosalicylic acid test for reducing sugar has been clarified previously, a t least in part. The 3,5-dinitrosalicylic acid is reduced to 3-amino-5-nitrosalicylic acid while, in the simplest instances, the aldehyde groups appear to be oxidized to carboxyl groups (4). The facts, however, that the equivalence between aminonitrosalicylic acid produced and sugar is not exact (4) and that different sugars yield different amounts of color ( 1 , 4 , 7), suggest that the chemistry of the test may actually hc appreciably more complicated. Such complications could conceivably be associated with the various decomposition reactions of sugars in alkaline solution ( 3 ) . If this explanation is correct, the reaction of the sugar aldehyde grouping with dinitrosalicylic acid could be viewed as competing with side reactions involving decomposition of the sugar. The effects of different concentrations of the various constitu- ents of the dinitrosalicylic acid reagent, and also of extraneous substances such as carboxymethylcellulose or citrate buffer, upon the amount of color produced and upon the destruction of glucose, as shown in the present study, could similarly be interpreted as effects upon the nature and degree of side reactions. The dinitrosalicylic acid reagent, in a form consisting only of dinitrosalicylic acid dissolved in strong alkali, has been used with apparent success for molecular n-eight measurement of starch breakdown products (?‘). This method depends upon the assumption that all higher oligosaccharides of the homologous series starting with maltose would produce equivalent amounts of color with the reagent. Actual studies of the reactions of members of homologous series with the dinitrosalicylic acid reagent, starting with the disaccharide, have not been reported, but u-ould be of considerable interest in the present connection. The principal virtue of the dinitrosalicylic acid test for reducing sugar lies in its great convenience compared to most other sugar tests, particularly u-hen large numbers of tests must be carried out. However, the factors discussed above must be given due consideration to avoid misinterpretation of results. LITERATURE CITED (1) Bell, D. J., Manners, D. J., Palmer, A,, J . Chem. Soc. 1952, 3760. (2) Brodersen, R., Ricketts, H. T., J . Lab. Clzn. Med. 34, 1447 (1949). (3) Gilman, H., “Organic Chemistry, Advanced Trratise,” 2nd ed., Vol. 2 , p. 1640, Wiley, Xew York, 1943. (4) Hostettler, F., Borel, E., Deuel, H., Helv. Chznz. d c t a 34, 2132 (1951). (5) Kolthoff, I. PII., Lingane, J. J., “Polarography,” Interscience, Kew York, 1946. (6) hlandels, G. R , Quartermaster Re search and Engineering Center, Natick, Mass., private communication. ( 7 ) bleyer, K. H., van der Wyk, A. J. A., Peng, C., Helv. Chznz. Acta 37, 1619 (1954). (8) XIiller, G. L., Blum, R., Glennon, W. E., Quartermaster Research and Engineering Center, Xatick, Mass., unpublished data. (9) Reese, E. T., Quartermaster Research and Engineering Center, Satick, illass., private communication. (10) Reese, E. T., Siu, R. G. H., Levinson, H. S., J . Bacterid. 59, 485 (1950). (11) Sumner, J. B., J . B i d . Chem. 47, 5 (1931). (12) Zbid.,62, 287 (1924-25). (13) Ibzd., 65, 393 (1925). (14) Sumner. J. B.. Sisler.’ E. B.. Arch. ‘ Bzochem. 4: 333 (1944). RECEIVED for review Sovember 4, 1957. Accepted September 23, 1958.