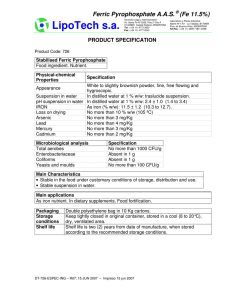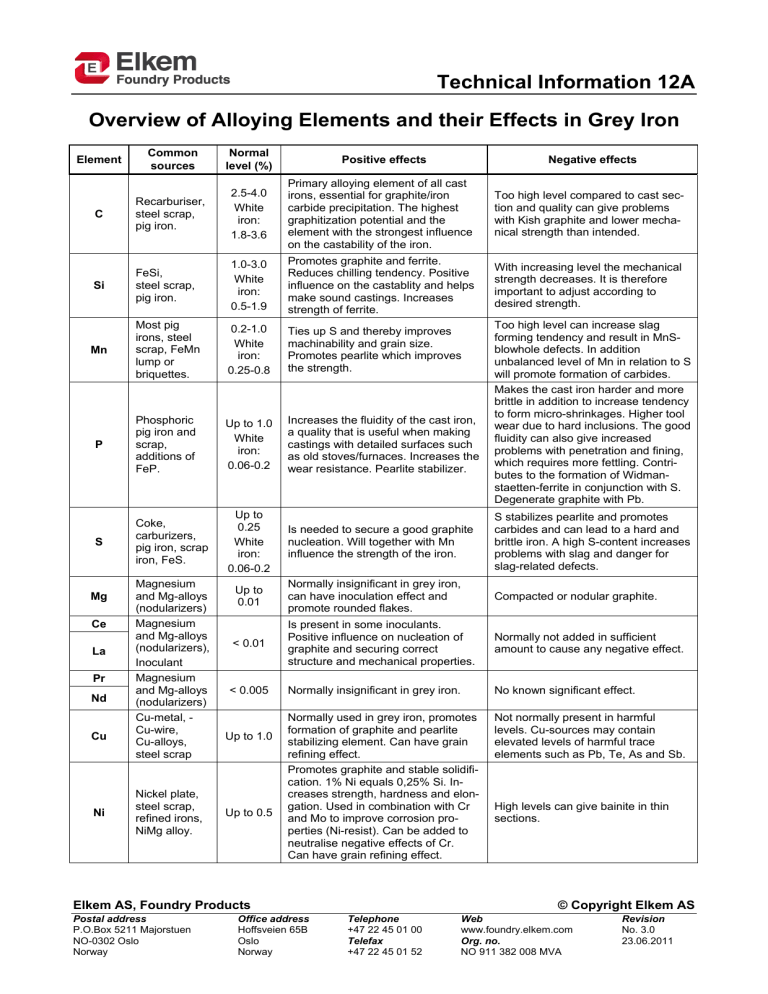
Technical Information 12A Overview of Alloying Elements and their Effects in Grey Iron Common sources Normal level (%) C Recarburiser, steel scrap, pig iron. 2.5-4.0 White iron: 1.8-3.6 Primary alloying element of all cast irons, essential for graphite/iron carbide precipitation. The highest graphitization potential and the element with the strongest influence on the castability of the iron. Too high level compared to cast section and quality can give problems with Kish graphite and lower mechanical strength than intended. Si FeSi, steel scrap, pig iron. 1.0-3.0 White iron: 0.5-1.9 Promotes graphite and ferrite. Reduces chilling tendency. Positive influence on the castablity and helps make sound castings. Increases strength of ferrite. With increasing level the mechanical strength decreases. It is therefore important to adjust according to desired strength. Mn Most pig irons, steel scrap, FeMn lump or briquettes. 0.2-1.0 White iron: 0.25-0.8 Ties up S and thereby improves machinability and grain size. Promotes pearlite which improves the strength. P Phosphoric pig iron and scrap, additions of FeP. Up to 1.0 White iron: 0.06-0.2 Increases the fluidity of the cast iron, a quality that is useful when making castings with detailed surfaces such as old stoves/furnaces. Increases the wear resistance. Pearlite stabilizer. S Coke, carburizers, pig iron, scrap iron, FeS. Up to 0.25 White iron: 0.06-0.2 Is needed to secure a good graphite nucleation. Will together with Mn influence the strength of the iron. S stabilizes pearlite and promotes carbides and can lead to a hard and brittle iron. A high S-content increases problems with slag and danger for slag-related defects. Up to 0.01 Normally insignificant in grey iron, can have inoculation effect and promote rounded flakes. Compacted or nodular graphite. < 0.01 Is present in some inoculants. Positive influence on nucleation of graphite and securing correct structure and mechanical properties. Normally not added in sufficient amount to cause any negative effect. < 0.005 Normally insignificant in grey iron. No known significant effect. Normally used in grey iron, promotes formation of graphite and pearlite stabilizing element. Can have grain refining effect. Promotes graphite and stable solidification. 1% Ni equals 0,25% Si. Increases strength, hardness and elongation. Used in combination with Cr and Mo to improve corrosion properties (Ni-resist). Can be added to neutralise negative effects of Cr. Can have grain refining effect. Not normally present in harmful levels. Cu-sources may contain elevated levels of harmful trace elements such as Pb, Te, As and Sb. Element Mg Ce La Pr Nd Cu Ni Magnesium and Mg-alloys (nodularizers) Magnesium and Mg-alloys (nodularizers), Inoculant Magnesium and Mg-alloys (nodularizers) Cu-metal, Cu-wire, Cu-alloys, steel scrap Nickel plate, steel scrap, refined irons, NiMg alloy. Up to 1.0 Up to 0.5 Positive effects Elkem AS, Foundry Products Postal address P.O.Box 5211 Majorstuen NO-0302 Oslo Norway Office address Hoffsveien 65B Oslo Norway Negative effects Too high level can increase slag forming tendency and result in MnSblowhole defects. In addition unbalanced level of Mn in relation to S will promote formation of carbides. Makes the cast iron harder and more brittle in addition to increase tendency to form micro-shrinkages. Higher tool wear due to hard inclusions. The good fluidity can also give increased problems with penetration and fining, which requires more fettling. Contributes to the formation of Widmanstaetten-ferrite in conjunction with S. Degenerate graphite with Pb. High levels can give bainite in thin sections. © Copyright Elkem AS Telephone +47 22 45 01 00 Telefax +47 22 45 01 52 Web www.foundry.elkem.com Org. no. NO 911 382 008 MVA Revision No. 3.0 23.06.2011 Technical Information 12A Element Cr Al Common sources Alloy steel, chromium plate, some pig irons, FeCr. Al-killed steel scrap, inoculants, Fe-alloys, light alloy components, Al-metal. Normal level (%) Up to 0.5 Up to 0.03 2 Positive effects Negative effects Give higher hardness and better wear resistance. Can help achieve finer distributed graphite. Promotes formation of iron carbides and can give problems with chill. Increased tendency for micro shrinkage. Important to achieve good inoculation effect. Can help tie up N and prevent N-related gas defects. Increases the solubility of H and thus the danger of H-related defects. Increases slag formation and danger of slag related defects. Higher degree of undercooling during solidification can give problems with incorrect graphite type and thus incorrect mechanical properties. Higher tendency for slag related defects. Can in combination with Al result in gas related defects. Increased chill, reduced cell count. Ti Some pig irons, some paints and vitreous enamels, CGiron returns, Ti-metal, FeTi Up to 0.15 Promotes ferrite and reduces chilling tendency. Refines the graphite structure. Can help tie up N and prevent gas related defects. Improved wear resistance. V Steel scrap, tool steel, some pig irons, FeV. Up to 0.10 Promotes pearlite and forms Vcarbides, which improve the strength and hardness. Improved wear resistance. Increases chilling tendency. Formation of V-carbides can have a negative impact on machinability. Up to 0.1 Forms Mo-carbides and has a weak pearlite promoting effect. Greatly improves the strength. 0,1% Mo can increase the strength by 4%. Improved properties at elevated temperatures and improved wear resistance. Mo-carbides can have a negative impact on machinability. Segregates heavily and results in carbides and more open structure at the grain boundaries. Problems with microporosities especially in combination with Cr and P. High levels can give bainite in thinner sections. Up to 0.01 Positive impact on graphite nucleation and gives a refined graphite structure. Can help tie up N. If too high levels are added it may promote undercooled graphite forms. Up to 0.15 Strong pearlite promoter and used deliberately to achieve fully pearlitic structure. Increases strength and hardness. Promotes undercooled D-graphite. Present at too high levels can lead to embrittlement of the iron Up to 0.02 Strong pearlite promoter and can be used to secure fully pearlitic matrix especially near cores. Can result in undesired graphite forms at elevated levels and will then have a negative impact on mechanical properties. Same as Pb. Up to 0.5 Refines eutectic cell size, graphite structure and the lamellae spacing of the pearlite matrix. Nb affects the nucleation of graphite and can hence support inoculation. The ultra hard NbC particles formed lead to a markedly increased wear resistance of the material. Strong carbide promoter. Up to 0.05 Strong pearlite promoter and can be used to secure fully pearlite matrix especially near cores. Can result in undesired graphite forms at elevated levels and will then have a negative impact on mechanical properties. Same as Pb. Ferrite promoter. No information on effect in grey iron No information on effect in grey iron Normally not used in grey iron. Promotes cementite in cups for thermal analysis (white curves). No information on effect in grey iron Strong carbide forming element difficult to control. Should be avoided if possible. Mo Refined pig irons, alloy steels, FeMo. Zr Inoculant Sn Sb Solder, tin plated steel scrap, bronze components, tin additions. Steel scrap, vitreous enamel scrap, bearing shells, Sbmetal. Nb HSLA steel scrap, Nbmetal, FeNb As Pig iron, steel scrap Zn Se Te Zinc coated steel scrap No known Up to 0.05 <0.001 Free-cutting copper, mould coatings, Up to 0.003 Technical Information 12A Element W Pb Common sources thermal cups. High speed tool steel. Old paints, some vitreous enamel, freecutting steel, terne plate, solder, petrol engine deposits. Normal level (%) Up to 0.05 Up to 0.005 Bi Inoculant, some mould coatings, core coatings. Up to 0.01 Ca Fe-alloys, nodulariser, inoculant. Up to 0.01 Ba Inoculants Up to 0.003 Co Tool steel Up to 0.02 Cd N 3 Plated bolts and screws. Coke, carburizers, core binders, steel scrap, NiFeMn. Positive effects Negative effects Promotes pearlite, but is not commonly used in grey iron. No known significant effect Pearlite promoter. Can result in undesired graphite such as Widmanstätten-graphite in heavy sections and mesh interconnected type in thinner sections, which has a negative impact on the mechanical properties. Increased chill. No known significant effect Promotes carbides and can result in undesired graphite forms such as Widmanstätten-graphite, which has a negative impact on the mechanical properties. Increased chill. Positive influence on nucleation of graphite and securing correct structure and mechanical properties. At the levels present in grey iron Ba has a positive influence on nucleation of graphite and securing correct structure and mechanical properties. Increases slag formation and danger of slag related defects. Increases slag formation and danger of slag related defects. No known significant effect No known significant effect No known significant effect Forms intercellular flakes. Up to 0.02 Promotes pearlite and increases strength. Up to 0.01 No known significant effect B FeB, vitreous enamel scrap, O Rusty scrap, pig iron. 4-6 ppm Essential for graphite nucleation. H Moist, rusty or oily charge materials, wet refractories or cores. <4 ppm None known. Increases chill. Can give gas related defectss if present in higher level (N>95ppm) depending on section thickness. Can give undesired compacted graphite. Promotes carbides especially in thin sections even at very low levels (B>0,001%). Too high level can give problems with gas porosity and increased slag formation. Too high levels of O in stream size inoculant could potentially reduce the effect of the inoculant and lead to slag defects. Subsurface pinholes and fissures, especially with higher Al or Ti levels. May increase shrinkage porosity. Promotes coarse graphite, and inverse chill when insufficient Mn present. References: BCIRA Broadsheet 192 ASM Specialty Handbook Cast Irons Giesserei Lexikon 2001 Legeringsämnenas inverkan på gjutjärnets egenskaper Sorelmetall Gusseisen mit Kugelgraphite Deutsches Roheisen Störelemente und schädliche Begleitelemente bei der Herstellung von Gusseisen mit Kugelgraphite Trace Elements in Gray iron This page intentionally left blank. Technical Information 12B Overview of Alloying Elements and their Effects in Ductile Iron Element Common sources Normal level (%) C Recarburiser, steel scrap, pig iron. 3.0-4.0 CGI: 2.5-4.0 Si FeSi, steel scrap, pig iron. 1.8-4.0 CGI: 1.0-3.0 SiMo: 4.0-6.0 Mn Pig iron, steel scrap, FeMn. 0.1-1.0 Ferritic grades: <0.20 Positive effects Negative effects Primary alloying element of all cast irons, essential for graphite precipitation. The highest graphitization potential and the element with the strongest influence on the castability of the iron. Promotes graphite and ferrite, reduces chill tendency. Positive effect on castability and helps make sound castings. Increases strength and as-cast ductility of ferritic grades. Positive impact on high temperature properties above 3% and is often combined with Mo, Ni and other elements. Promotes pearlite formation and improves hardenability. P Some pig iron Increases fluidity. Stabilizes pearlite, and steel Up to 0.03 increases hardness and strength. scrap, FeP. S Coke, carburizers, some pig iron and steel scrap, mould sand, FeS. Up to 0.03 >0.03 with Essential for graphite nucleation and a desulphu- recommended minimum is 0.005%. rization Mg Magnesium and Mg-alloys (nodularizers) The most essential element for ductile 0.03-0.06 irons. Deoxidiser and desulphuriser, enabling nodular graphite growth. Elkem AS, Foundry Products Postal address P.O.Box 5211 Majorstuen NO-0302 Oslo Norway Office address Hoffsveien 65B Oslo Norway Too high level compared to cast section and quality can cause problems with irregular graphite forms and flotation, giving lower mechanical strength. With increasing content the ductile-tobrittle transition temperature increases giving lower impact strength. At elevated levels Si and C-content has to be adjusted to avoid graphite flotation, increased slag formation and problems with mould filling. A higher casting temperature is needed. Carbide forming element that can segregate to grain boundaries and result in carbides, which can reduce mechanical strength. This negative effect can partly be reduced by increasing Si-content in thin sections, but if possible the level of Mn should be kept low in thicker section castings and other elements should be selected to control the matrix structure. Increases the ductile-to-brittle transition temperature. Can promote shrinkage. Should be kept as low as possible, damaging in ductile irons above 0.03%. Decreases ductility and result in a more brittle matrix by introducing micro porosities. Forms low melting phosphide eutectic at the grain boundaries in addition to promoting pearlite. Negative effect can be partly counteracted by addition of Si. Can have detrimental effect on mechanical properties which heat treatment can only partly cure. Increases the ductile-to-brittle transition temperature. If not neutralised, flake graphite will form instead of nodular graphite. At levels >0,020% increases Mg consumption and may give problems with slag formation and reduced consistency of the Mg-treatment. Damaging to structure and properties unless balanced by manganese. Highly volatile, high treatment temperatures decreases Mg yield. High levels compared to S can give carbides, micro porosities and increased slag formation. Turbulent mould filling can give dross (slag stringers). © Copyright Elkem AS Telephone +47 22 45 01 00 Telefax +47 22 45 01 52 Web www.foundry.elkem.com Org. no. NO 911 382 008 MVA Revision No. 3.0 23.06.2011 Technical Information 12B Element Common sources Ce Mg-alloys, Misch metal, Ce-metal, Inoculant. La Mg-alloys, Misch metal, La-metal, inoculant. Pr Nd Cu Mg-alloys, Misch metal. Mg-alloys, Misch metal. Cu-metal, Cu-wire, Cu-alloys, steel scrap. Ni Nickel plate, some steel scrap, refined irons, NiMgalloys. Cr Alloy steel, Cr-plate, some pig irons, FeCr. Al Ti V Normal level (%) 2 Positive effects Deoxidiser and desulphuriser, enabling nodular graphite growth. Effective inoUp to 0.02 culant, more fade resistant than Mg. Neutralises subversive elements: Ti, Al, Sb, Pb, Bi and Sn, total <0.03%. Deoxidiser and desulphuriser, enabling nodular graphite growth. Effective inoculant, stronger than Ce, more fade re<0.015 sistant than Mg. Neutralises subversive elements such as Pb, As, Ti, Sb and Bi. <0.010 Similar to Ce. <0.010 Similar to Ce. Up to 1.5 Up to 0.2 Ni-resist: 18-36 Up to 0.1 Al-killed steel scrap, inoculants, Up to 0.2 ferroalloys, light alloy components, Al-metal. Some pig irons, paints and vitreous Up to 0.2 enamels, CGI returns, T-metal, FeTi. Steel scrap, tool steel, Up to 0.02 some pig irons, FeV. Promotes pearlite without promoting carbides and increases strength and hardenability. Promotes formation of graphite, but weaker than Si. Stabilises austenite. Increases strength without making the ferrite more brittle and can be used as a Si-subsititute in ferritic grades. Increases hardenability especially in combination with Mo and/or Cu. Improves corrosion resistance and high temperature properties if alloyed correctly. Slightly grain refining effect. Reduces chill. Pearlite stabilising element, but hardly used as that. Increases hardenability and wear resistance. Commonly used in austenitic ductile iron to improve oxidation and corrosion properties in high temperature alloys. Raises nodule count. Improves graphite nucleation and promotes formation of ferrite. Neutralizes nitrogen. May be neutralized by cerium. Promotes pearlite. Improves yield and ultimate tensile strength in heat treated ferritic ductile iron with limited reduction of impact and elongation. Promotes pearlite. Negative effects High levels can give carbides in thin sections and chunky graphite in thick sections. Chunky counteracted by controlled additions of Sb, Pb and Sn. Carbide stabilizing due to segregation. High levels can give carbides in thin sections and chunky graphite in thick sections. Chunky graphite can be counteracted by addition of Sb, Pb and Sn. Carbide stabilizing due to segregation. Similar to Ce, but no significant effect at normal levels. Similar to Ce, but no significant effect at normal levels. Impairs ferritisation and can give more brittle ferrite. Levels >1% can result in degeneration of the graphite form. Selection of Cu-source is important as it may contain harmful trace elements such as As, Pb, Te and Sb. In presence of Cu the negative effect of these elements are often enhanced. Promotes intercellular flake graphite in combination with Ti or Pb. Promotes chunky graphite at elevated levels and high carbon equivalent. Strong carbide promoter. Increases chilling tendency. Promotes stable carbides and segregates heavily to grain boundaries. Synergetic effect with Mn. Promotes H-pinholes above >0.015%. Gives slag related defects if present at elevated levels. Detrimental to nodular graphite above approx. 0.08%. Should be kept as low as possible in ductile iron. Reduces nodule count and impairs nodular graphite formation at elevated levels. Promotes H-pinholes especially in combination with Al. May be counteracted by rare earth metals. Forms very stable carbides that may have a negative impact on later machinability. Retards annealing. Promotes chill. Technical Information 12B Element Common sources Mo Refined pig irons, alloyed steels, FeMo. Zr Precondition, inoculant Sn Sb Nb As Zn Se Te W Pb Bi Normal level (%) 3 Positive effects Promotes pearlite and increases hardenability. Increases hardness and yield strength through solid solution hardening. Combined with Ni and Cr for improved properties at high temperatures. Improves graphite nucleation and can Up to 0.01 assist in tying up N to avoid gas related defects. Up to 0.1 SiMo: 1.0-2.0% Solder, tin Strong pearlite promoter. Can be used plated steel in combination with Cu to stabilise scrap, bronze Up to 0.15 pearlite content in heavy casting components, sections. Improves strength. Sn-metal. Steel scrap, Strong pearlite promoter. Added to vitreous heavy section castings to counteract enamel scrap, Up to 0.01 chunky graphite (50-100 ppm). Can bearing help neutralise rare earths if necessary. shells, Sbmetal. HSLA steel Increased mechanical properties and scrap, NbUp to 0.01 corrosion behaviour. metal, FeNb Strong pearlite promoter. Added to heavy section castings to counteract Pig iron, steel Up to 0.01 chunky graphite. Can help neutralise scrap. rare earths if necessary. Improves nodular graphite shape. Zinc coated steel scrap Ca Ferroalloys, nodularizers, inoculants. Ba Ba-bearing inoculants. Co Tool steel. Segregates strongly and forms stable carbides at the grain boundaries. Promotes carbides. Can promote compacted graphite at elevated levels. Carbide former. Impairs formation of ferrite. Embrittles ductile irons above 0.08 %. Can give intercellular flake graphite. Can result in degenerated graphite (spiky graphite and lamellar graphite at grain boundaries) if present in absence of rare earths. Strong carbide promoter. Inhibits nodularity in absence of rare earth’s. Strong carbide promoter. No information on effect in ductile iron Can result in degenerated graphite (spiky graphite and lamellar graphite at grain boundaries) if present in absence of rare earths. Strong carbide promoter. Can be controlled by Ce. Environmental and health problems during melting. Can condensate on the induction coil leading to break down of induction furnace. Decreases Mg-yield and can cause degenerated graphite at levels above 0.04%. No information on effect in ductile iron Can control pinholes at max 0.003%. Negative effects can be counteracted by Mg and Ce. Strong carbide forming element difficult to control. Should be avoided if possible. Promotes pearlite. Carbide promoter. Level should be kept as low as possible as negative effect can occur even at level lower than the detection limit of Pb. Promotes pearlite. Effects on graphite in ductile irons are neutralized by rare earths. Promotes intercellular flake graphite which can have negative effect on mechanical properties. Promotes carbides. Causes degenerated nodular graphite forms. Should be avoided or melting process should be selected to handle ZnUp to 0.05 contaminated scrap. Effectively removed by base iron stirring at high temperatures. No known <0.002 Free-cutting copper, mould <0.005 coatings, thermal analysis samples. High speed Up to 0.03 tool steel. Old paints, some vitreous enamel, freeUp to cutting steel, 0.005 solder, petrol engine deposits. Bi-bearing inoculants, Up to 0.01 mould coatings containing Bi. Negative effects Can help give rounder and smaller graphite nodules, but is best used in combination with RE to avoid incorrect graphite structure. Increases number of nodules. Improves spheroidization of graphite nodules. Improves graphite nucleation. Up to 0.01 Reduces chilling tendency and promotes graphite. Improves graphite nucleation and Up to reduces fading. Reduces chilling 0.003 tendency and promotes graphite. Up to 0.02 No known significant effect Promotes iron carbides and can give undesirable graphite forms such as Widmanstätten graphite and by that reduced mechanical properties. Excessive nodule count can cause shrinkage. Increases slag formation and danger of slag related defects. Can also promote chunky graphite if combined with high levels of Si and/or Ni. No known significant effect Carbide promoter. Technical Information 12B Common sources Element Cd 4 Normal level (%) Plated bolts and screws. Positive effects No known significant effect B Vitreous >5 ppm gives more ferrite. A level that enamel scrap, Up to 0.01 can be difficult to control and keep FeB. consistent in ordinary production. N Coke, carburizers, core binders, steel scrap, NFeMn. O Rusty scrap, pig iron. H Moist, rusty or oily charge materials, wet refractories or cores. Up to 150 ppm Not normally a problem in ductile iron since Mg also ties up N. promotes pearlite. Defects can be neutralised by Al, Ti and Zr. Base iron: 4-6 ppm Essential for graphite nucleation. Final iron: <1 ppm < 4 ppm None known. Negative effects Forms intercellular flakes. Strong carbide promoter >10 ppm. Limits Cu’s pearlite forming. Reduces hardness. Normally it is recommended to keep B-content as low as possible. Can result in nitrogen fissures (holes) at higher levels (>85-110 ppm) dependent on section thickness and increase shrinkage porosity. Can give undesired compacted graphite. Too high level will require higher addition of MgFeSi and will give problems with slag generation and gas porosity. High levels of O in in-mould grades are especially critical. Can give H pinholes, especially with higher Al or Ti levels. Can increase shrinkage porosity. References: BCIRA Broadsheet 192 ASM Specialty Handbook Cast Irons Giesserei Lexikon 2001 Legeringsämnenas inverkan på gjutjärnets egenskaper Sorelmetall Gusseisen mit Kugelgraphite Deutsches Roheisen Störelemente und schädliche Begleitelemente bei der Herstellung von Gusseisen mit Kugelgraphite Review Ductile Iron: Fifty years of continuous development Effects and Neutralization of Trace Elements in Gray, Ductile and Malleable iron