Compressive Mechanical Properties of Human Cancellous bone after Gamma Irradiation
Anuncio

Compressive Mechanical Properties
of Human Cancellous Bone afler Gamma Irradiation*
II Y M It I 1A IU. J. AN GE RSON. M.D. t. JOYCE IN. KEY A K. B.S.M.E.{. AND HA RR Y B. SKI NNE R, M. D., I•I1. D. t.
SAN FRANt’lSC’O. C’ALI FORN i A
lii ve.xti¿‹iIi‹›it |›‹’rf‹›rttt‹•r/ al hit’ Defi›urtm‹’itt ‹›f Orih‹›yue‹lic Snryery, Sch‹›ol ‹›f Me‹licine, University ‹›f C‹ilif‹›rniu, San Fr‹ut‹isc‹›
ABSTRACT: The effect of gamma irradiation on the
mechanical properties of human bone was examined.
Specimens of cancellous bone were cut from the proximal epiphyseal region of fresh-frozen tibiae and divided
into control and irradiated groups according to anatomical region. The irradiated groups were exposed to
10,000, 31,000, 51,000, or 60,000 gray (1.0, 3.1, 5.1, or 6.0
megarad). The specimens were tested in compression to
failure to determine failure stress, strain to failure, and
elastic modulus.
Failure stress und elastic modulus were found to be
proportional to the square of the density and were
normalized with respect to this property. Significant
dilTerences in normalized failure stress (p < 0.001) and
normalized elastic modulus (p = 0.003), when compared
with the values for matched control specimens, were
found only for the specimens that had been irradiated
with 60,000 gray (6.0 megarad).
CLINICA L RELEYANCE: These data suggest that
compressive failure stress and elastic modulus ofcqncellous tibial bone in humans decrease significantly when
the bone is irradiated with 60,000 gray (6.0 megarad).
However, these properties did not decrease significantly
when the accepted dose for sterilization before allografting (25,000 gray [2.5 megarad]) was used.
The use of allograft bone has increased since the
early 1980"s' " ". and various methods of sterilization
and preservation have evolved‘’. Such diverse practices
as sterile as opposed to clean harvesting, storage with
freezing or freeze-drying, and treatment with ethylene
oxide or irradiation for sterilixation are being used. The
effects of these various methods of processing on the
*N ti bcnc l’it s in tiny form have bcc n received or will he received
from a c‹imnicrci‹il p‹irt v related directly or indirectly to lhc subject of
this article. Funds were received in tt›tal or partial support of the
research or clinical studv presented in 1 his arlicle. The funding sources
were the Department t›f Veterans A ffairs. Rehabilitation Research and
Development Sc rvicc: the Depart ment of Orthopaedic Surge ry. Univcrsit y of Califtirnia. S‹in Francisco›: the San Francisco Foundation for
Research and Education in Orthopaedic Surgery: and the University
t›f California. San Francisco. Ti.ssuc Bank.
tDcp‹irlmcnt uf C3rthopacdic Surgery. University of C”alifornia.
San Francisco›. Schotil of Medicine ( tJ471 ). San Francisco. California
94143-0728.
Rc habilitat ion Research and Development Service, Department
t›f Veterans A fl"‹iirs Medical Ccntc r. San Francisco. California 94121.
VOL 74-A. M() l JñMl: 19U2
biomechanical properties of human bone are largely
unknown.
There is growing concern about the sterility and
safety of allograft bone‘. Although these fears may be
ungrounded, many physicians prefer so-called sterilised
bone, and studies have shown that high doses of radiation
(more than the conventional 25,00U gray [2.5 megarad])
may be necessary to eradicate the human immunodeficiency virus’. Irradiation to a dose of 25,00t1 gray (2..5
megarad) has been shown to ensure 10(I per cent sterilization of bacteria in specimens of cortical bone' that arc
less than five centimeters deepz. The use of large structural allograft bone continues to increase, but the biomechanical propertics of this bone in compression, aftcr
administration of even small doses of radiation, are
largely unknown. These properties are important because many reconstructions involve cancellous or corticocancellous grafts that will be loaded primarily in a
compressive mode.
Changes in the biomechanical properties of bone
after irradiation have been investigated. Komender
found a 20 per cent decrease in the compressive strength
of cortical bone from the human femoral diaphysis following the administration of a dose of 60,000 gray (6.0
megarad). However, he found no significant change in
strength after administration of 30,000 gray (3.0 megarad). Triantafyllou et al., studying the cortical bone of
bovine tibiae, found that torsional strength decreased by
50 to 75 per cent after fresh-freezing and irradiation with
30,000 gray (3.0 megarad).
We studied the compressive biomechanical properties of human cancellous bone, with and without irradiation, because of the common use of cancellous allografts.
Materials and Methods
The proximal portion of matched pairs of tibiae from
two human male cadavera (ages at death, fifty-one and
sixty-seven years) were obtained from the University of
California, San Francisco, Tissue Bank. These specimens
had been rejected from the donor pool either because of
superficial contamination during procurement or because they did not meet the age criteria established by
the American Association of Tissue Banks. The specimens wcre frozen to —80 degrees Celsius within twentyfour hours after death and were stored at —20 degrees
747
748
M. J. ANDERSON, J. H. K E YA K, AND H. B. SKI N NER
2 cm
3AD
2 cm
1
The arrangement and orientation of the specimens
Celsius. Each tibia was examined roentgenographically,
and no abnormalities were detected. The bone was processed after it had been cleared with studies of donor sera,
in accordance with guidelines established by the American Association of Tissue Banks, and after approval had
been given by the University of California, San Francisco,
Biosafety and Human Studies Committees
The tibial plateaus were cleaned of all soft tissue, and
polymethylmethacrylate forms were constructed about
the tibial shafts to aid in the cutting. Rough cuts were
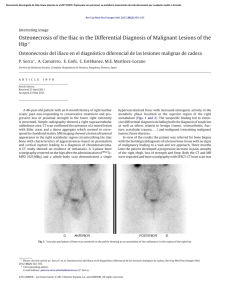
made with a twenty-five-centimeter (ten-inch) table-saw.
These cuts were located just distal to subchondral bone
and then again about three centimeters distal to the
initial cuts (Fig. 1). Next, the specimens were cut to a
thickness of two centimeters with a low-speed saw (Isomet;Buehler, Lake Bluff Illinois) that was equipped with
a diamond blade (Fig. 1).These blocks then were cut into
one by one by two-centimeter specimens Care was taken
to maintain the tibial orientation of the samples, so that
compressive loading during testing would be in the same
direction as the physiological load (Fig. 1, inset).
The specimens of bone were divided into ten control
groups and ten groups for irradiation. The strength and
elastic modulus of tibial epiphyseal bone varies more in
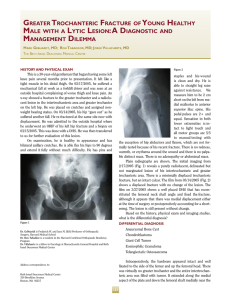
the medial-lateral direction than in the anterior-posterior direction’. Therefore, the groups were defined as
shown in Figure 2, with the medial-lateral location being
identical for all specimens within a group. Additionally,
the specimens that were to be exposed to radiation were
alternately chosen from the right and left tibiae. For
example, the medial specimens from the right tibia (IC)
served as the control group for the experimental (irradiated) medial specimens from the left tibia (IX).The next
group of samples, the mid-medial specimens from the
right tibia (2X), were subjected to irradiation, while the
mid-medial specimens from the left tibia (2C) served as
the control group.
The level of irradiation to which each experimental
group would be exposed was sequentially assigned as the
bone was processed. The first experimental group (IX)
received 10,0fD gray (1.0 megarad); the second experimental group (2X), 31,0fD gray (3.1 megarad); the third
(3X), 51,0fD gray (5.1 megarad); and the fourth (4X),
60,000 gray (6.0 megarad). The fifth group (5X) received
10,000 gray (1.0 megarad);the sixth group (6X), obtained
from the other donor, received 31,000 gray (3.1 megarad); and so on.
The total number of specimens from both donors at
lcm
lcm
RIGHT
ANT£RIOR
FIG. 2
The locations of the irradiated (X) and control specimens (C).
THE JOU RNAL OF BONE AND JO INT SURGERY
€”I JM Pk I- SSI V I- M I:€”I1AN I €”A L PROPE RTI ES OF H LT M A N LAN €”E LLO LV S BON E A FTE R Ci AM M A I R R A D I AI 10 N
Nt›minal IrraJiaiit›n
M r‹lJ
Cray
Failure
Strcss
Density
(¿/cnt’)
740
Strain lo
Failure
(Per certs)
1.3 + (1.4
II.2'i2 z 0.0ñ4
( io)
?.l
3.39 1 2.tJ5
(10)
27t) + 24()
2.14 + 2.() I
2lX) 1 I4f)
(1.241 + (I.()fi9
IN.241 + 0.069
fi. 1
().241 + U.040
( I ))
II
W)UA)
”A ve rt›yc ‹Ind stanJarJ Jcviat ion. Thc numhcr of specimens is in
I .3 1).4
parcnthescs.
each level of irradiation was between six and ten. A total
of thirty control and thirty-one irradiated specimens
wc re obtained. The experimental groups were exposed
lo gamma radiation from a cobalt-60 source (through the
Northern California Transplant Bank, San Francisco. California). The samples were rotated 180 degrees at the
halfway point to ensure uniform dosage. The bone tissue
was kept frozen in dry ice during the irradiation process.
Testing was done at 21 degrees Celsius. An MMED
servohydraulic mechanical testing machine (Matco, La
Canada. California) with a 2200-newton load-cell was
used for all testing. Cross-head displacement was measured with the internal transducer of the testing machine,
log [FAILURE STRESS (NPa)1
I .2
3
log [DENSITY lg/cc)l
Fici. 2
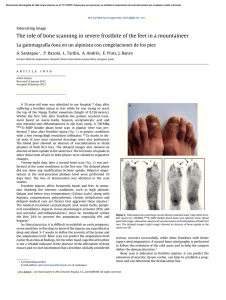
A I ypical lt›aJ-Jisplaccmcnt curve. Thc z.cro position is arhitrary.
and the progress of the tests was simultaneously recorded on an analog plotter. The specimens were tested
in an unconstrained fashion, with the marrow left in
situ’ "'. The samples to be tested were thawed to room
temperature in a water bath and measured with a micrometer. They were pre-loaded in compression to five
newtons and then pre-conditioned to a displacement of
0.1 millimeter at a rate of 0.2 millimeter per second for
five cycles. This method of conditioning was necessary to
obtain repeatability in the low load, elastic range, as
viewed on the analog plots. This is in agreement with the
method of Linde et al.". After conditioning, the samples
were loaded to failure at a strain rate of 1 per cent per
second. The force-displacement data were digitally recorded at ten hertz. The stiffness of the testing machine
also was measured so that the data could be corrected
for machine compliance (see Appendix).
After testing, the samples were defatted in four fourhour baths of 200-proof ethyl alcohol, followed by four
four-hour baths of ethyl ether (purified for fat extraction) (Fischer Scientific. Fair Lawn, New Jersey). The
specimens were dried for a minimum of six hours and
were weighed. To determine the bulk density (p), dry
weight was divided by bulk volume.
Anul ysis of Dara
The raw data consisted of the data on load versus
displacement, the height (h) and cross-sectional area (A)
of the specimen, and the ultimate failure load (F ,). F.i,
was defined as the maximum compressive load that had
been reached before the specimen collapsed. Linear regression analysis of the digital data was performed on the
linear region of the load-displacement data for each
sample. The derived slope (k„„,i) was the stiffness of the
over-all system — that is, bone plus machine. The slope
750
M. J. AN DE R SO N, J. H. K EYA K. AN D H. B. SK IN N E R
TAB LE 11
STt I191:NT T-Tt-ST Rs st! I:i‘S f €JR D I: N'›lTY. FA ISURE STR2Ss. STRAI N T€J FAILURE. AND ELAS’i‘ic’ Moot t.t is i ‹iu Fot o LI: vial .s or [ RR.\ IU A l’lt iN
Density
Nominal Irradiation
Mrad
Gray
1.(1
3.1
5.1
6.(1
for Cent of
Control Value
8.3.fi
95.fi
101
87..5
It).G8i
31.fXitl
fil.(CIO
fit1.HiU
Failure Stress
P Value
Per Cent of
Control Value
0.166
(1.655
0.964
0.417
78.9
58.5
102
23.9
Strain to Failure
P Value
Per Cent of
Control Value
0.462
0.f›69
0.960
0.029
HXJ
9tJ.4
IU
82.7
Mc›dulus
P Value
Per Cent of
C‹introl Value
P V‹iluc
1).989
II.fiI14
I).38b
(1.199
fi2.1
lt)7
ltXl
28.4
II.2fi4
IN.54
11.994
t1.IU
and displacement were then corrected for machine com- examined, and regression analyses were performed. The
pliance to obtain the stiffness of each specimen (k , ) and mechanical test data were then normalixed according to
specimen displacement (see Appendix). A plot of load the results of these regressions. The Student t test was
versus displacement for a typical specimen is shown in used to compare the normalized data for each level of
Figure 3.
irradiation with those for the matched controls.
Failure stress (S), strain to failure (c.„), and elastic
Results
modulus (E) were calculated with the following equations, which assume that these properties are linear: S =
The density, failure stress, strain to failure, and elastic
F.„/A, e.„ = Fu,/ k ,.:h, and E = ku,.:h/A.
modulus for the control and experimental specimens are
shown in Table I. No significant differences in compresStatistical Analysis
sive failure stress were found between the specimens that
The Student t test was used to compare the failure had been irradiated with 1tJ,00t), 31.(I()t). or 51,(IU() gray
stress, strain to failure, and elastic modulus for each (1.0, 3.1, or 5.1 megarad) and the control specimens. The
group of irradiated specimens with those values for the compressive failure stress was significantly different, comrespective control group (Primer of Biostatistics soft- pared with the control value, only for the specimens that
ware package; New York, McGraw Hill, 1988). The Stu- had been irradiated with 60,t1t1O gray (6.IN megarad) (p <
dent t test was also used to assess differences in failure 0.03) (Table II). Differences in compressive strain were
stress. strain to failure, and elastic modulus of all control not significant even when the specimens had been irra(non-irradiated) specimens. The differences between the diated with 60,00tJ gray (6.0 megarad). The decrease in
right and left tibiae of the same donor were determined, modulus approached significance (p = 0.06) at 60,000
as were the differences between donors. To assess the gray (6.0 megarad). There was no significant difference
effect of density on the measured properties. linear and in the density between the irradiated groups and the loglog plots of failure stress, strain to failure, and elastic control groups, and no significant difference in the den- modulus
versus density for the control specimens were sity, failure stress, or elastic modulus of the control spec-700
—L00—
-500
1
-400—
@ —30C
0
0
-200—
-100
0
0 . 00 -0.25 -0.50 -0.75 -1.00 -1 25 -1 50
POSITION lmm)
Flu. 4
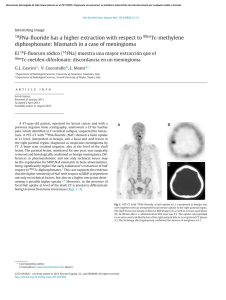
Log failure stress versus log density for all control specimens
imens was seen between donors or between the right and
left tibiae of the same donor.
Plots of failure stress and elastic modulus versus
density revealed non-linear relationships for these specimens. This finding is in agreement with that of others’
who reported power function relationships between density and strength and between density and modulus.
Correlation of log failure stress with log density (Fig. 4)
and of log modulus with log density for the control
specimens showed regression slopes of 2.09 + 0.23 (p <
0.001) and 2.08 + 0.34 (p < ().(J0l ), respectively. The
intercepts were log (51.3) and log (3890), respectively.
Therefore, the relationships between failure stress. elastic modulus, and density were assumed to be: S (MPa) =
51.3 p' and E (MPa) = 3590 p', where p is in grams per
cubic centimeter. To account for the effects of specimen
density on the measured mechanical properties, the failure stress and elastic modulus were normalized by division by the square of the density. The Student t tests of
these data revealed significant differences only for the
specimens that had been irradiated with 60.tD0 gray (6.0
megarad) for both normalized failure stress (p < 0.001)
THE JOURNAL OF BONE AN D JOINT SU RG ERY
I tl M P R 2 SS1 V I M I- t’H AN 1 C’A L P RO P ERT I ES OF H U MAN CAN CE LLOU S BO N E A FT L R €i A M M A I R RA D I A I I €J N
75 1
TABLE 111
Failure Stress/Density'
(MP‹d|flniIj-’)
Nt›min‹ll I rrtiJi‹itit›n
MraJ
Cray
El‹istic Modulus/Density
(MP ii/|y/tttll-’ )
Mean + S.D.”
Mean + S.D.”
tl.474
I).H 7
I .I)
42IXI+??IX)
4(XX)+lfi(X)
fil+17
()Glh
11.fifi2
4H + 11
(1.79fi
â.l
fi3 + 12
h.(I
I ñ(X) + 4(X)
19 + 3
* . D. = standard dcviatit›n.
and normalized elastic mOdulus (p = 0.003) (Table I I I).
Discussion
amine these biomechanical properties in relation to various levels of gamma irradiation.
Our results indicate that the compressive failure
strcss and elastic modulus of allografts of human cancellous tibial bone decrease significantly when the bone has
been irradiated with 60,(8)O gray (f›.0 megarad). These
properties did not decrease significantly in the specimens
that had been subjected to lower levels of irradiation.
Therefore, there is no evidence that irradiation with
25,000 gray (2.5 megarad), the accepted dose for sterilization, affects the mechanical properties of proximal
tibial allografts.
The failure stress and elastic modulus of the nonirradiated specimens of this study. which were from cadavcra of men. fifty-one and sixty-seven years old at the
time of death, compare favorably with data from the
literature for epiphyscal bone from fresh-frozen tibiae’.
The quadratic relationship between elastic modulus and
density determined in our study differs from the findings
of Carter and Hayes. which indicate a cubic relationship.
However. this difference may be due to the use of diskshaped specimens and test conditions of uniaxial strain
Appendix:
in the previous study. The relationship of failure stress
Correction
of
Mechanical Test Data
with density at a strain rate of 1 per cent per second in
for
Machine
Compliance
our study is the same as that reported by Carter and
Hayes. Their value of S/p'of 51.6 megapascals per (gram
Force-displacement data were recorded for direct
per cubic centimeter)' at a strain rate of 1 per cent per platen-to-platen loading of zero to 1000 newtons at a rate
second is nearly identical to the value of 51.3 megapas- of twenty newtons per second for six cycles with use of
cals per (gram per cubic centimeter)' that we obtained the previously described transducers. Regression analyfrom the intercept of the log S versus log p regression. ses on the data were performed to obtain the stiffness of
Furthermore. no significant difference in the properties the testing machine (k „„„,:), which was 5350 + 130 newof thc control specimens was seen between donors or tons per millimeter (average and standard deviation).
between the right and left tibiae of the same donor. The stiffness of each specimen, k „,., was then determined
Therefore, we believe that our specimens are repre- from: k ,.: = (kg,. ,.. k„„.,)/(k „. ,.. — k„„„i). The increase in
sentative of the donor population.
stiffness after correction for machine compliance ranged
Many centers use as much as 25,000 gray (2.5 mega- from 3 per cent for the least-stiff specimen (k,„.: = 160 rad)
of gamma irradiation for sterilization of clean or
newtons per millimeter) to 100 per cent for the most-stiff
sterilcly harvested allografts. Despite this use of irradia- specimen (k,„ : = 5330 newtons per millimeter). The distion, we found no reported data on the compressive placement data were corrected for machine compliance
biomechanical properties of human cancellous allograft in a similar manner: corrected displacement = measured
bone after irradiation. This study was performed to exdisplacement — (load/kg.,. ,.:).
Rel'erences
1. American Asso‹i»ii«» »r issue Banks: Tel hiiical Mnttiusl for Surgical Boiir Buitkiiig. McLean. Virginia. American Association of Tissue
Banks. 1987.
2. Bright, R. W.: Gambill, V. M.; and Smsnh, J. D.: Ouantitativc effects of sterilising irradial ion on human bonc. ln Ti.s.sue Grafls iii
Rev ‹cii.strut tit'‹• Sim,qer v, pp. l9f›-2()1. Edited by P. Karatzas and N. Triantafyllou. Athens. Greek Atomic Energy Commission. 1981.
VOL. 74-A. NO. 5. I I I N E 1992
752
4.
6.
7.
9.
1 l.
12.
14.
15.
M . J. A N DE R SON. J. H . K E YA K. AN D 11. B. S K I N N E R
Brighi, R. W.; Smarsh, J. D.; and Gambill, V. M.: Sterilization of human bone by irradiation. I n Osteo‹ li‹›iu:lr‹il All‹›griifis. Bioluz v, l1‹itikiii z,
uitil Cliiti‹al A fiplit uiiun.s. pp. 230-231. Edited by G. E. Friedlacndcr, H. J. Mank in. and K. W. Sell. Bt›ston, Little, Brown. 1983.
Buck, B. E.; Malinin, T. I.; and Brown, M. D.: Bone transplantation and human immunodeficiency virus. An cstim‹itc of risk of .acquired
immunodc ficicncy syndrome (A I DS). Cfirt. Orthop., 240: 129- 13f›, 1989.
Carter. D. R., and Hayes, W. C.: The compressive behavior of hone as a two-phase porous structure. J. Borne riiirt J‹›iiit .hurt.. fi°7-A: 9fi4-9G2,
Oct. 1977.
COttWW .; TomFord, W. W.; Hirsch, M. S.; Schooled, R. T.; and tVtankin, H. J.: Effects of gamma irradiation on H I V- 1 in a ht›nc allograft
model. Truiis. Grth‹›y. Res. Son’., 1.5: 225. 1990.
Goldstein, S. A.: The mechanical propcrties of trabccular bone: dependence on an atomic location and function. J. Bi‹›me‹’li. . 211: 1 tJfifi1 llf›1, 1987.
Head, W. C.; Berklscich, F. M.; Malinin, T. I.; and Emerson, R. H., Jr.: Proximal femoral allografts in rcvisitin tut‹il hip urthrt›plasty. C“/iii.
Orlliof›., 225: 22-36. 19b7.
Komender, A ndrzej: I nfiuencc of preservation on some mechanical prope rtics of human havcrsi‹in fionc. Mun•r. ñfcr/. fr›/‹›iiri, A: 1317. l97b.
Linde, F., and Hvid, I.: The effect of constraint on the mechanical behaviour of trahccular bone specimc ns. 7. fiiornt•‹ ii., 22: 4Hfi-49t), 1989.
Linde, r.: ‹sslhgen, C. B.; Hvid, 1.; and Pongsoipetch, B.: Mechanical properties of trabecular fionc bv a n‹›n-dcstruci ive ct›mprcssi‹in
testing approach. Eiig. Meâ.. 17: 23-29, 1988.
Oakeshott, R. D.; Morgan, D. A. F.i Zukor, D. 7.; Rudan, J. F.; Brooks, P. J.i and Gross, A. E.: RcvisitJn tt›lal hip arthrcpl‹isty with t›ssct›us
allograft reconstruction. A clinical and roentgenographic analysis. Clin. Orihoy., 22fi: 37-61, 1987.
Skinner, H. B.: Alternatives in the selection of allograft bone. Orthopedics, 13: 843-84d. 1990.
Tomford, W. W.: Ploetz, J. E.; and M•nkin, H. J.: Bone allografts of femoral heads: procurement and storage. J. d‹iile hurt Smith .Surg., hA- A:
534-537, April l9Hb.
Tomford, W. W.; Thongphasuk, Jarunee; Mankin, H. J.; and Ferraro, M. J.: Froxcn musculoskeletal .illogr‹ifts. A studY tif the clinical
incidence and causes of infection associated with iheir use. J. Bone and Joinl Surg., 72-A: 11.37- 1.143. Sept. l99(1.
Trianlafyllou, N.; Soliropoulos, E.; and Triantafyllou, J. N.: The mechanical properties of the lyophylizcd and irr‹idi‹itcd bonc gral"ts. A‹’ru
Orthofi. Belgi‹-u, 41 (Supplement 1 ): 33-44. 1975.
TH E JOU R NAI. OF BONE AN f3 JO I NT SU RG E RY