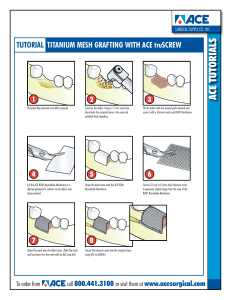
Materials Science & Engineering C 99 (2019) 620–630 Contents lists available at ScienceDirect Materials Science & Engineering C journal homepage: www.elsevier.com/locate/msec Surface engineering of titanium-based implants using electrospraying and dip coating methods T Akbar Javadia, Atefeh Solouka, , Masoumeh Haghbin Nazarpakb, , Fatemeh Bagheric ⁎ ⁎ a Department of Biomedical Engineering, Amirkabir University of Technology (Tehran Polytechnic), Tehran, Iran New Technologies Research Center (NTRC), Amirkabir University of Technology (Tehran Polytechnic), Tehran, Iran c Department of Biotechnology, Faculty of Chemical Engineering, Tarbiat Modares University, Tehran, Iran b ARTICLE INFO ABSTRACT Keywords: Titanium implants Surface modification Electrospraying Dip coating Titanium and its alloys due to their low density, good mechanical and biological properties are of the most common orthopedic metals. One of the main challenges regarding to titanium implants is their loosening after long term implantation in patient's body. Many methods such as alteration in surface topography with focus on improving osseointegration or biocompatibility in overall are supposed to overcome this issue. In this research, titanium surface topography is altered via electrospraying a solution of titanium salt, carrier polymer (polyvinylpyrrolidone) and solvents. The dip coated samples in the same solution are prepared and investigated as control. The electrosprayed or dip coated samples were pyrolysised in furnace at 500 °C to remove polymeric components. Then the stabilized microstructures on the surfaces were evaluated via scanning electron microscopy (SEM), water contact angle (WCA) measurement, X-ray diffraction (XRD) and atomic force microscope (AFM). Also, in order to study the bioactivity of modified samples, they were immersed in simulated body fluid (SBF) and their precipitates were studied. The cellular investigations were done by studying the cell morphology, MTT and alkaline phosphatase (ALP) activity assays. The results showed improvement in bioactivity and cellular response for DP3 and SP15 more than other samples implying the promising potential of these two approaches for titanium implant surface modification. 1. Introduction [6,7]. Chouirfa et al. studied the effect of different solutions to decrease bacterial infections on the Titanium surface according two main parts: surface modification and coatings (chemical or physical) [8]. Janson et al. also provided antibacterial activity by soaking the surfaces of Titanium samples in hydrogen peroxide [9]. Loosening is a consequence of implant poor integration with the surrounding bone tissue remains as one of the major obstacles for permanent orthopedic implants [5]. Also, nanoscale surface topography and biomaterial roughness are considered as crucial as surface chemical composition for tissue acceptance and cell viability [10]. Hamlekhan et al. have reported that surface modification and presence of nanoscale features on the surface of implants can enhance the growth of osteoblast bone-forming cells. The rationale behind this is the similarity of nanometer features with the osteoblast biological environment [11]. Different surface modification procedures are studied to improve the performance of medical implants. Some of them are laser surface modification [12], thermal oxidation [13], sand blasting [14], sol-gel coating [15], physical vapor deposition (PVD) procedures [16], chemical vapor deposition (CVD) [17], plasma spray [18] and the Titanium (Ti) and its alloys are widely used in orthopedic implant materials because of their appropriate properties, such as relatively low modulus, good fatigue strength, formability, machinability, corrosion resistance, and biocompatibility. Ti and its alloys like other biomaterials must meet all the essential criteria, especially the physical, chemical, and mechanical properties, to have a desirable function in the human body. Designing biomaterials is often challenging to fulfill all functional requirements, therefore fabricating a material with adequate and acceptable bulk properties along with additional steps to modify surface is a common approach [1,2]. So, various surface modification processes in micro and nanoscale are studied and investigated [3,4]. Biomaterial surface plays an important role in the body response. In the case of titanium based implants, the normal manufacturing steps usually create a non-uniform and rather poorly defined oxidized and contaminated surface layer. In order to take these “native” surfaces for biomedical applications surface modification seems essential [5]. Infection and loosening are two major challenges of orthopedic implants ⁎ Corresponding authors at: Amirkabir University of Technology (Tehran Polytechnic), Iran. E-mail addresses: [email protected] (A. Solouk), [email protected] (M. Haghbin Nazarpak). https://doi.org/10.1016/j.msec.2019.01.027 Received 13 August 2018; Received in revised form 23 December 2018; Accepted 7 January 2019 Available online 25 January 2019 0928-4931/ © 2019 Published by Elsevier B.V. Materials Science & Engineering C 99 (2019) 620–630 A. Javadi et al. polymer (polyvinylpyrrolidone), appropriate solvents, and secondly dip coating using the same solution. Then, the physical, chemical and biological properties of these altered surfaces are investigated and compared with unmodified Ti surface. Table 1 Electrospraying optimum parameters. Feeding rate Needle to substrate distance (d) Applied voltage 100 mm 150 mm 10 kV 15 kV 0.5 mL/h 0.5 mL/h 2. Materials and methods 2.1. Materials and reagents Table 2 Dip coating optimum parameters. Solution volume to surface area ratio (mL/mm2) 3/100 3/100 Polyvinylpyrrolidon (PVP, Mw = 1,300,000 g/mol) as the carrying polymer, Ti(IV) n-butoxide (TBut) as the alkoxide precursor, and all the solvents such as acetic acid (AcA), N,N dimethylformamide (DMF, ≥99) and Isopropanol were purchased from Merck company. A mixture of N,N dimethylformamide and Isopropanol was used in 1:1 mass ratio. All reagents were used without further purification. Commercially pure titanium with a purity of 99.9% was used as substrate. Simulated body fluid (SBF) for evaluating the bioactivity of specimens, was provided with mixing NaCl, NaHCO3, KCl, K2HPO4.3H2O, MgCl2.6H2O, HCl (1 M), CaCl2, Na2SO4, (CH2OH)3CNH2 (Tris), all obtained from Merck company. All Titanium samples (0.1 mm thick plates, 99.7% purity [ATI CPTi]) were cleaned with 15 min ultra-sonication in distilled water then washed three times with distilled water and air dried [23]. Dip coating time (h) 2 3 Table 3 Coding the specimens. Specimens Description CTRL SP10 SP15 DP2 DP3 Control specimen without any surface modification Electrosprayed (V = 10 kV, d = 100 mm, f = 0.5 mL/h) Electrosprayed (V = 15 kV, d = 150 mm, f = 0.5 mL/h) Dip coating specimen (2 h) Dip coating specimen (3 h) 2.2. Preparation of solution procedures that result in titanium dioxide structures on titanium surface [19]. Two of these surface modifications created titanium dioxide nanotubes on the surface via anodizing method [20] and titanium dioxide nanofibers on the surface via electrospinning [21,22]. One simple and cost effective approach to form microfibers/microparticles on the Titanium surface is using Titanium salts and electrospinning/electrospraying techniques. Ti(IV) n-butoxide salt has been used to create nanofibers structure on Ti surface via electrospinning method for biomedical applications [23]. Recent studies about the electrospraying of TiO2 nanoparticles on the Ti surface have been done for some other applications such as light sensitive solar cells [24] and as coating membrane for aluminum substrate [25] but to the best of our knowledge there is no report in using mentioned Ti salt in order to cover the Ti implant surface with microparticles. In this research, titanium surface topography is altered via two approaches, firstly electrospraying a solution of titanium salt, carrier Solvents with the weight percentage of 43% (Isopropanol), 43% DMF and 2% AcA were mixed together. Next, PVP (8 wt%) was added and the solution stirred for 4 h with stirring speed of 400 rpm and then the titanium salt (TBut) (4 wt%) was added and stirred with speed of 250 rpm for 14 h. At the end, a milky solution was obtained for both electrospraying and dip coating test [22,23]. 2.3. Preparing the specimens In this research, two methods (electrospraying and dip coating) were used to create the microstructured morphology on titanium samples. The electrospraying/electrospinning apparatus parameters were used to prepare the TiO2 microfibers before [23], by changing the parameters and evaluating the results and surface morphologies, two optimal groups of electrospraying parameters were obtained (Table 1). It should be noted that in electrospraying method, titanium plates were Fig. 1. SEM micrographs of CTRL specimen at different magnifications, a) 5000×, b) 10,000×. 621 Materials Science & Engineering C 99 (2019) 620–630 A. Javadi et al. Fig. 2. SEM micrographs of electrosprayed specimens a) SP10 (5000×), b) SP10 (10,000×), c) SP15 (5000×), d) SP15 (10,000×). used as the substrate. The specimens prepared by electrospraying, were then exposed to air under the fume hood in order to evaporate the solvents. The second method was dip coating the titanium plates in the same solution which was used in electrospraying. Two main parameters of this method is dip coating time and solution volume/surface area ratio. This ratio was kept constant and four different dip coating times selected and the surface morphologies were evaluated, then two optimal parameters were used to prepare the specimens for carrying on the tests (Table 2). After preparing the specimens with two different methods, heat treatment was done for three main purposes: the PVP was cleaned from the surface. Specimens coding is presented in Table 3. 2.4. Characterization of the surfaces To study the morphology of the surfaces before and after modification and also after immersing in the simulated body fluid (SBF), the scanning electron microscopy was used. The solution used for the surface modification process included a polymer (PVP) and 3 different solvents, in addition to titanium salt. In order to assure removing these four materials during the heat treatment, X-ray diffraction peaks of the specimens with wavelength of 1.54187 Å, working voltage 40 kV and working current 30 mA were studied. Wettability is one of the important factors affecting the cell adhesion; so that more wettability of the surface, more adhesion of the osseous cells to the surface [27,29]. In order to investigate the wettability of the specimens, water contact angles (WCA) were measured. In this method, a drop of water was put on the surface with a specified needle and then imaging with a camera was done. Then the drop contact angle was measured with image-j software. Then atomic force microscope (AFM) was used to study the surface morphology and roughness with high precision. AFM microscope evaluates the surface by the means of a sharp needle at the free end of a lever. The forces between the needle and surface, results in deviation of the lever and a detector measures the amount of deviation 1- Burning the PVP and getting a pure structure of TiO2 on the surface. 2- Achieving the TiO2 anatase crystal structure. 3- Strengthening the coating/substrate adhesion. It should be noted that the reason of choosing the anatase structure for TiO2 is its desirable physical and biological properties compared to rutile or amorphous structure [26]. The heat treatment program was as follows; heating the specimens from room up to 500 °C at a heating rate of 2 °C/min at air atmosphere, and soaked for 2 h and then cooled back to room temperature with the cooling rate of 2 °C/min [23]. After taking the specimens out of the furnace, the ash, resulting from burning 622 Materials Science & Engineering C 99 (2019) 620–630 A. Javadi et al. Fig. 3. SEM micrographs of dip coating specimens a) DP2 (5000×), b) DP2 (10,000×), c) DP3 (5000×), d) DP3 (10,000×). Fig. 4. X-ray diffraction spectra of samples a) SP15, b) DP3. during the surface scanning [30]. In this project a noncontact AFM microscope with a silicon needle, 150 kHz frequency and 2 Hz scanning speed was used to study the surface properties of the titanium specimens, before and after modification by both methods and then the results were investigated with Q- port software. 2.5. Bioactivity investigation The sample in vitro bioactivity was investigated by their apatiteforming ability after immersing in simulated body fluid (SBF). This solution was prepared with a protocol suggested by Kokubo et al. [28]. 623 Materials Science & Engineering C 99 (2019) 620–630 A. Javadi et al. Fig. 5. Topographic pictures of AFM microscope a) CTRL, b) SP10, c) SP15, d) DP2 and e) DP3. to decreasing the concentration of salts in SBF solution (because of precipitation on the specimens), the SBF solution was substituted every 2 days with a fresh one. Table 4 Average roughness numbers for electrospraying and dip coating specimens. Roughness (Rt) (nm) – 427.8 305.2 345.9 554.7 ± ± ± ± 11.9 6.26 1.51 5.76 Roughness (Ra) (nm) Specimen – 81.3 ± 2.2 70.1 ± 1.5 70.8 ± 1.4 105.7 ± 1.8 CTRL SP10 SP15 DP2 DP3 2.6. Cellular investigation (1) 2.6.1. Mesenchymal stem cells (MSCs) isolation Rabbit bone marrow was harvested from the tibia of 3–4 months old New Zealand white rabbits with the procedure were approved by the Ethics Committee of Tarbiat Modares University (Tehran, Iran). Bone marrow was mixed with DMEM (Dulbecco's Modified Eagle's Medium) containing 100 IU/mL penicillin, 100 IU/mL streptomycin and 15% FBS (Fetal Bovine Serum) and incubated at 37 °C and 5% CO2. We then incubated the cultures until confluency and replaced the medium every other day. Passaged-3 cells were used for following experiments [32]. After dip coating of the specimens in SBF, the samples were air dried. SEM images, EDX mapping of Ca and P, Ca/P ratio, and the amount of increased weight was provided. It should be noted that due 2.6.2. Three-dimensional cultures First, the specimens were cut into small size of 5 × 5 mm and sterilized by 70% ethanol for 10 min. Then, 15,000 passaged-3 MSCs In this method, the required volume of the solution was calculated by Eq. (1). At this equation, Vs is the required volume for dip coating (mL) and Sa is the area of the surface in contact with solution (mm2) [26,30,31]: Vs = Sa /10 624 Materials Science & Engineering C 99 (2019) 620–630 A. Javadi et al. Fig. 6. Water drop contact angle test results, (n = 3) a) CTRL (82.6 ± 0.4), b) SP10 (54.2 ± 1.7), c) SP15 (25.3 ± 1.0), d) DP2 (36.9 ± 0.8) and e) DP3 (13.4 ± 0.4). were suspended in 20 μl DMEM medium and placed on top surfaces of the specimens. Before the cultures were provided with medium, they were preincubated at 37 °C for 30 min for cell attachment. The cultures were then provided with DMEM medium containing 15% FBS, 10 IU/ mL penicillin, and 10 IU streptomycin and incubated in an atmosphere of 5% CO2 and temperature of 37 °C. 2.6.3. Cell morphology on the surfaces Morphology of MSCs cultured on the surfaces was assessed using SEM images. After 3 days, the samples were washed with PBS and fixed with 2.5% glutaraldehyde. The samples were then dehydrated in a concentration gradient of ethanol solutions (30%, 50%, 70%, 80%, 90%, and 100%), coated with gold and visualized at scanning electron microscope. 2.6.4. MTT assay The attachment and viability of the cultured MSCs on the different surfaces was evaluated using MTT (3–(4, 5-dimethylthiazol-2-yl) 2, 5diphenyltetrazolium bromide) (Sigma, USA) assay. Succinctly, 3 days after culture, the fresh medium containing MTT solution (5 mg/mL in PBS) was added in a 5:1 ratio. After incubation at 37 °C for 2 h, the medium was removed and the precipitate was dissolved in dimethyl Fig. 7. Weight increase for 1 and 7 days after immersion in SBF. 625 Materials Science & Engineering C 99 (2019) 620–630 A. Javadi et al. Fig. 8. SEM pictures of a) CTRL (250×), b) CTRL (1000×), c) SP15 (250×), d) SP15 (1000×), e) DP3 (250×) and f) DP3 (1000×) specimens after 1 day immersion in SBF. sulfoxide (DMSO). Absorbance was measured at the wavelength of 545 nm and cell viability was calculated as the percent value relative to the untreated surface [33]. dip coated. All of these specimens were observed by SEM microscopy under two magnifications, 5000× and 10,000×. According to Fig. 1, the surface of the specimens was covered with passive natural oxide layers before modification. Also, this surface had micron size pores without any ordered morphology. As can be seen in Fig. 2, modified surface of SP15 had higher density of TiO2 particles covering its surface compared with SP10. Surface modified specimens by dip coating (DP) method also are shown in Fig. 3. At first glance, the amount of particles (or the layer of particles) created on the surface for DPs looks more than SP specimens. Moreover, TiO2 particles on the surface of DP samples could join together in better way and cover the large natural pores on titanium surface. Comparing SEM images of DP2 and DP3, it can be realized that increasing immersion time could results in precipitation of more particles on the surface. In order to characterize the TiO2 phase and being sure of removing the solvents and carrier polymer from the surface after heat treatment, the prepared specimens were analyzed with X-ray diffraction which is shown in Fig. 4. Only Ti and TiO2-anatase peaks were observed in the X- 2.6.5. Alkaline phosphatase (ALP) assay To investigate osteogenic differentiation, alkaline phosphatase (ALP) activity, was measured using ALP kit (BioVision, USA). Briefly, cell seeded specimens in osteogenic media were washed with PBS and lyses with lysis buffer. The cell lysate (200 μL) was then mixed with 300 μL of p-nitrophenyl phosphate (pNPP) substrate solution. After incubation at 37 °C for 45 min, 100 μL of stop solution was added to the above to stop the reaction and the absorption at 405 nm was measured spectrophotometrically [34]. 3. Results and discussion 3.1. Surface characterization There were three different specimens: control, electro-sprayed and 626 Materials Science & Engineering C 99 (2019) 620–630 A. Javadi et al. Fig. 9. SEM pictures of a) CTRL (250×), b) CTRL (1000×), c) SP15 (250×), d) SP15 (1000×), e) DP3 (250×) and f) DP3 (1000×) specimens after 7 days immersion in SBF. ray spectra of both SP and DP samples. In fact, other four additive used during dip coating or electrospraying (isopropanol, DMF, acetic acid and PVP) escaped during the heat treatment in the furnace because of their lower evaporation temperature (i.e. 82.6 °C, 152 °C, 118 °C and 150–180 °C, respectively) [35]. In order to investigate the surface topography, AFM analysis was performed on all samples and results are shown in Fig. 5 and Table 4. As it was expected, untreated Ti surface had nearly smooth surface and Qport software could not report any roughness number for it. It is known that roughness number can be explained with parameters such as Ra and Rt. Ra is the difference between average of valleys and peaks; and Rt is the difference between highest peak and deepest valley on the surface [36]. Comparing SP10 and SP15 results showed smoother surface for SP15 (Ra = 70.1 nm) with more covered pores as the consequence of monolayer formation and better surface coverage with TiO2 particles which confirmed SEM results. In the case of dip coated samples, DP3 has rougher surface (Ra = 105.7 nm) than DP2 (Ra = 70.8 nm) as the immersion time was increasing. In all four modification treatments, the obtained surface showed higher roughness rather than smooth control but SP15 and DP3 had highest surface roughness, 70.1 and 105.7 nm, respectively. This microstructure may be advantageous for biomedical applications, because it can augment bone intergrowth and speed up interfacial bonding between the implant and the natural bones. It is because of the rapid growth of HAp crystals due to the high Ca content [37–39]. Water contact angle measurements of all samples are shown in Fig. 6. It is known that decreasing the WCA indicates increasing in water tendency to spread on the surface then wettability of the surface also increased [40]. Wettability is affected by both chemical composition and topography of the surface. Since in the present study chemical composition of all specimens was the same, the difference in WCA is related to modifications of the surface topography. According to the Fig. 6, the WCA of control specimen is about 82.6° which is significantly decreased after performing both surface modification processes (electrospraying and dip coating) to 54.2°, 25.3°, 36.9° and 13.4° for SP10, SP15, DP2 and DP3, respectively. These results are in agreement with AFM data and show the effect of enhancing surface roughness on increasing wettability. In all modified samples the surfaces are coarser 627 Materials Science & Engineering C 99 (2019) 620–630 A. Javadi et al. environment is an important factor to assay metallic implant osteointegration. In the current study, it is evaluated by soaking the samples in SBF in two time intervals (i.e. 1 and 7 days) as presented in Fig. 7. Due to the high corrosion resistance of titanium salt, the bioactivity can be measured by the mass change in the samples before and after immersion. As expected from AFM and WCA results, SP15 and DP3 showed the most significant weight increase in both time intervals. Therefore, their SEM images in two mentioned time periods and EDX analysis were presented in Figs. 8, 9, and 10, respectively. The surface coverage with a precipitated layer is evident and EDX investigations revealed that the layers are composed of calcium and phosphorous. Furthermore, the Ca/P ratio for control, SP15 and DP3 in day 1 were 1.59, 1.60, 1.58 and 1.60, 1.67 and 1.64 after passing 7 days. From the literature, it has been mentioned that Ca/P ratio for hydroxyapatite composition is about 1.67 [40]. The formation of hydroxyapatite layer was started by immersing the titanium samples in SBF and allowing the hydroxyapatite particles to coat the surface [41]. Comparing the results of days 1 to 7 showed increasing trend by enhancing soaking time which is in agreement with Eslami et al. [42]. Moreover, the Ca/P ratio of both SP15 and DP3 after 7 days immersion in SBF are very close to hydroxyapatite (i.e. 1.67) This effect plays a key role on bone ingrowth and on the implant fixation bone-like apatite layer, which was also in agreement with Kokubo and Takadama's research [29]. From these findings, it can be concluded that the Ca/P ratio of both SP15 and DP3 after 7 days immersion in SBF are very close to hydroxyapatite. 3.3. Cellular investigation The number of viable cells on modified surfaces in compare with control group was quantified using MTT assay (Fig. 11). According to results, the viable cells showed significant increase in contact with both DP3 and SP15. No significant difference has been found in the cell vitality comparison between the DP2 and SP10 groups and control. The interaction of samples with Mesenchymal stem cells (MSCs) was investigated after 3 days as presented in Fig. 12. It can be seen that in all samples the MSCs cells has proper cells attachment but in comparison with unmodified samples, modified titanium samples show more cell flatting and ECM secretion of adhered cells is observable, especially for DP3 and SP15 that the cells formed nearly a monolayer on their surfaces. As reported by other researchers, the cell activity on implants is affected by surface properties such as morphology, roughness, chemical composition, and wettability (surface free energy) [43]. So the results of MTT and cell adhesion confirmed AFM, wettability and Fig. 10. Ca and P weight percent on the surface of specimens after immersion in the SBF solution for 1 and 7 days. than control one (Fig. 5) which may cause smaller WCA. It can be concluded that Ti-Salt precipitate is more hydrophilic comparing to Titanium oxide. 3.2. Bioactivity The ability to encourage apatite growth in a physiological Fig. 11. Cell viability of MSCs on the specimens after 3 days. 628 Materials Science & Engineering C 99 (2019) 620–630 A. Javadi et al. a b c d e Fig. 12. SEM images of cell morphology on the surface of a) CTRL, b) DP2, c) DP3, d) SP10 and e) SP15 specimens. with the control (p < 0.01). The influence of surface roughness on differentiation of MSC is reported previously [44,45]. 4. Conclusion In this study, titanium surface modification was reported via two different coating methods under four conditions. SEM and AFM results showed that unmodified smooth and porous surface of Ti completely changed to rough microstructured surface especially for DP3 and SP15 samples. XRD results revealed that only Ti and TiO2-anatase phase were formed on the surface of both SP and DP samples. WCA of control specimen was about 82.6° which significantly dropped to 25.3° and 13.4° for SP15 and DP3, respectively. Also, the bioactivity of modified samples was studied after immersion in SBF, and the apatite growth ability along with weight increase were found predominantly in SP15 and DP3. MSCs were also cultured on the surface of all samples but the cell proliferation and ALP activity for DP3 and SP15 were again more than other samples. These findings may imply the potential of electrospraying and dip coating methods to promote bone growth and matrix mineralization rate on the surface of modified titanium implant as a result of surface chemical and structural alterations in samples. Fig. 13. ALP activity results on day 14. bioactivity assays findings. At last, differentiation of MSCs to osteoblastic phenotype was evaluated by alkaline phosphatase (ALP) activity. ALP activity was evaluated in three replicates on day 14 and reported in Fig. 13. ALP activity significantly increased in SP15 and DP3 samples in comparison 629 A. Javadi et al. Materials Science & Engineering C 99 (2019) 620–630 References solar cells, Aerosol Sci. Technol. 47 (12) (2013) 1302–1309. [25] S.U. Halimi, A. Noor Fitrah, S.N. Ismail, M. Nazli Naim, Electrospray deposition of titanium dioxide (TiO2) nanoparticles, 5th Nanosci. Nanotech. Symposium (NNS2013), vol. 1586, AIP Publishing, 2014No. 1. [26] H. Jie, W. Zhou, X. Zhou, X. Zhong, X. Zhang, P. Wan, B. Zhu, W. Chen, The anatase phase of nanotopography titania plays an important role on osteoblast cell morphology and proliferation, J. Mater. Sci. Mater. Med. 19 (11) (2008) 3465–3472. [27] M.A. Surmeneva, C. Kleinhans, G. Vacun, P.J. Kluger, V. Schönhaar, M. Müller, S.B. Hein, A. Wittmar, M. Ulbricht, O. Prymak, C. Oehr, R.A. Surmenev, Nanohydroxyapatite-coated metal-ceramic composite of iron-tricalcium phosphate: improving the surface wettability, adhesion and proliferation of mesenchymal stem cells in vitro, Colloids Surf. B: Biointerfaces 135 (2015) 386–393. [28] P. Eaton, P. West, Atomic force microscopy, Oxford University Press, 2010. [29] T. Kokubo, H. Takadama, How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27 (15) (2006) 2907–2915. [30] Z. Zhenyu, J. Qin, J. Ma, Electrophoretic deposition of biomimetic zinc substituted hydroxyapatite coatings with chitosan and carbon nanotubes on titanium, Ceram. Int. 41 (7) (2015) 8878–8884. [31] N.B. Li, G.Y. Xiao, B. Liu, Z. Wang, R. Zhu, Y. Lu, Rapid deposition of spherical apatite on alkali–heat treated titanium in modified simulated body fluid at high temperature, Surf. Coat. Technol. 301 (2015) 121–125. [32] E. Nejati, V. Firouzdor, M. Eslaminejad, F. Bagheri, Needle-like nano hydroxyapatite/poly (l-lactide acid) composite scaffold for bone tissue engineering application, Mater. Sci. Eng. C 29 (2009) 942–949. [33] H. Ghorbani, A. Abdollah-zadeh, F. Bagheri, A. Poladi, Improving the bio-corrosion behavior of AISI316L stainless steel through deposition of Ta-based thin films using PACVD, Appl. Surf. Sci. 456 (2018) 398–402. [34] M. Aghajanpoor, S. Hashemi-Najafabadi, M. Baghaban-Eslaminejad, F. Bagheri, S. Mohammad Mousavi, F. Azam Sayyahpour, The effect of increasing the pore size of nanofibrous scaffolds on the osteogenic cell culture using a combination of sacrificial agent electrospinning and ultrasonication, J. Biomed. Mater. Res. A 105 (2017) 1887–1899. [35] S. Liel, C.B. Stanley, D. Harries, Properties of polyvinyl pyrrolidone in a deep eutectic solvent, J. Phys. Chem. A 120 (19) (2016) 3253–3259. [36] E.S. Gadelmawla, M.M. Koura, T.M.A. Maksoud, I.M. Elewa, H.H. Soliman, Roughness parameters, J. Mater. Process. Technol. 123 (1) (2002) 133–145. [37] M.-S. Kim, J.-J. Ryu, Y.-M. Sung, One-step approach for nano-crystalline hydroxyapatite coating on titanium via micro-arc oxidation, Electrochem. Commun. 9 (2007) 1886–1891. [38] F. Liu, F. Wang, T. Shimizu, K. Igarashi, L. Zhao, Fabrication of hydroxyapatite coatings on AZ31 Mg alloy by micro-arc oxidation coupled with sol–gel treatment, Surf. Coat. Technol. 199 (2005) 220–224. [39] L. Scheideler, J. Geis-Gerstorfer, D. Kern, F. Pfeiffer, F. Rupp, H. Weber, H. Wolburg, Investigation of cell reactions to microstructured implant surfaces, Mater. Sci. Eng. C 23 (2003) 455–459. [40] E. Carlos Nelson, Y. Oshida, J. Henrique, C. Limad, C. Alberto Muller, Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque, J. Mech. Behav. Biomed. Mater. 1 (3) (2008) 234–242. [41] A.R. Rafieerad, M.R. Ashra, R. Mahmoodian, A.R. Bushroa, Surface characterization and corrosion behavior of calcium phosphate-base composite layer on titanium and its alloys via plasma electrolytic oxidation: a review paper, Mater. Sci. Eng. C 57 (2015) 397–413. [42] N. Eslami, R. Mahmoodian, M. Hamdi, Nadia Mahmoudi Khatir, M.K. Herliansyah, Ali Reza Rafieerad, Study the synthesis, characterization and immersion of dense and porous bovine hydroxyapatite structures in Hank's balanced salt solution, J. Miner. Met. Mater. Soc. 69 (4) (2017) 691–698. [43] L. Hao, X. Fu, T. Li, N. Zhao, X. Shi, F. Cui, C. Du, Y. Wang, Surface chemistry from wettability and charge for the control of mesenchymal stem cell fate through selfassembled monolayers, Colloids Surf. B: Biointerfaces 148 (2016) 549–556. [44] E.M. Lee, K. Smith, K. Gall, B.D. Boyan, Z. Schwartz, Change in surface roughness by dynamic shape-memory acrylate networks enhances osteoblast differentiation, Biomaterials 110 (2016) 34–44. [45] B.D. Boyan, A. Cheng, R. Olivares-Navarrete, Z. Schwartz, Implant surface design regulates mesenchymal stem cell differentiation and maturation, Adv. Dent. Res. 28 (1) (2016) 10–17. [1] A. Solouk, B.G. Cousins, H. Mirzadeh, A.M. Seifalian, Application of plasma surface modification techniques to improve hemocompatibility of vascular grafts: a review, Appl. Biochem. Biotechnol. 58 (5) (2011) 311–327. [2] L. Xuanyong, P.K. Chu, C. Ding, Surface modification of titanium, titanium alloys and related materials for biomedical applications, Mater. Sci. Eng. R 47 (3) (2004) 49–121. [3] A. Cimpean, E. Vasilescu, P. Drob, I. Cinca, C. Vasilescu, M. Anastasescu, V. Mitran, S. Iulian Drob, Enhancement of the electrochemical behaviour and biological performance of Ti–25Ta–5Zr alloy by thermo-mechanical processing, Mater. Sci. Eng. C 38 (2014) 127–142. [4] R.J. Narayan, Big possibilities for small scale implants, Mater. Today 16 (6) (2013) 204–205. [5] W. Lin, H. Tan, Z. Duan, B. Yue, R. Ma, G. He, T. Tang, Inhibited bacterial biofilm formation and improved osteogenic activity on gentamicin-loaded titania nanotubes with various diameters, Int. J. Nanomedicine 9 (2014) 1215–1230. [6] H. van de Belt, D. Neut, W. Schenk, J.R. van Horn, H.C. van der Mei, H.J. Busscher, Infection of orthopedic implants and the use of antibiotic-loaded bone cements: a review, Acta Orthop. Scand. 72 (6) (2001) 557–571. [7] D. De Leonardis, A.K. Garg, G.E. Pecora, Osseointegration of rough acid-etched titanium implants: 5-year follow-up of 100 minimatic implants, Int. J. Oral Maxillofac. Implants (1999) 384–391. [8] H. Chouirfa, H. Bouloussa, V. Migonney, C. Falentin-Daudré, Review of titanium surface modification techniques and coatings for antibacterial applications, Acta Biomater. 83 (2019) 37–54. [9] Oscar Janson, Satwik Gururaj, Shiuli Pujari-Palmer, Marjam Karlsson Ott, Maria Strømme, Håkan Engqvist, Ken Welch, Titanium surface modification to enhance antibacterial and bioactive properties while retaining biocompatibility, Mater. Sci. Eng. C 96 (2019) 272–279. [10] J. Park, S. Bauer, K. von der Mark, P. Schmuki, Nanosize and vitality, TiO2 nanotube diameter directs cell fate, Nano Lett. 7 (6) (2007) 1686–1691. [11] A. Hamlekhan, C. Takoudis, C. Sukotjo, M.T. Mathew, A. Virdi, R. ShahbazianYassar, T. Shokuhfar, Recent progress toward surface modification of bone/dental implants with titanium and zirconia dioxide nanotubes, J. Nanotechnol. Smart Mater. 1 (2014) 1–14. [12] Y.S. Tian, C.Z. Chen, S.T. Li, Q.H. Huo, Research progress on laser surface modification of titanium alloys, Appl. Surf. Sci. 242 (1) (2005) 177–184. [13] H. Guleryuz, H. Cimenoglu, Surface modification of a Ti–6Al–4V alloy by thermal oxidation, Surf. Coat. Technol. 192 (2) (2005) 164–170. [14] J. Guo, R.J. Padilla, W. Ambrose, I.J. De Kok, L.F. Cooper, The effect of hydrofluoric acid treatment of TiO2 grit blasted titanium implants on adherent osteoblast gene expression in vitro and in vivo, Biomaterials 28 (36) (2007) 5418–5425. [15] H.W. Kim, H.E. Kim, J.C. Knowles, Fluor-hydroxyapatite sol–gel coating on titanium substrate for hard tissue implants, Biomaterials 25 (17) (2004) 3351–3358. [16] A. Ewald, S.K. Glückermann, R. Thull, U. Gbureck, Antimicrobial titanium/silver PVD coatings on titanium, Biomed. Eng. Online 5 (1) (2006) 22. [17] S. Varanasi, J. Ni, and D. M. Nelson, Chemical vapor deposition process for depositing a titanium oxide coating, U.S. Patent No. 20,160,076,144. 17 Mar. 2016. [18] X. Liu, P.K. Chu, C. Ding, Surface modification of titanium, titanium alloys, and related materials for biomedical applications, Mater. Sci. Eng. R 47 (3) (2004) 49–121. [19] Y. Bin, M. Mahjouri-Samani, C.M. Rouleau, D.B. Geohegana, K. Xiao, Low temperature synthesis of hierarchical TiO2 nanostructures for high performance perovskite solar cells, Phys. Chem. Chem. Phys. 18 (2016) 27067–27072. [20] K.S. Brammer, C.J. Frandsen, S. Jin, TiO2 nanotubes for bone regeneration, Trends Biotechnol. 30 (6) (2012) 315–322. [21] S. Khorshidi, A. Solouk, H. Mirzadeh, S. Mazinani, J.M. Lagaron, S. Sharifi, S. Ramakrishna, A review of key challenges of electrospun scaffolds for tissue-engineering applications, J. Tissue Eng. Regen. Med. 10 (9) (2015) 715–738. [22] J. Ik Lim, B. Yu, K. Mi Woo, Y. Lee, Immobilization of TiO2 nanofibers on titanium plates for implant applications, Appl. Surf. Sci. 255.5 (2008) 2456–2460. [23] C. Dumitriu, A.B. Stoian, I. Titorencu, V. Pruna, V.V. Jinga, R.M. Latonen, J. Bobacka, I. Demetrescu, Electrospun TiO2 nanofibers decorated Ti substrate for biomedical application, Mater. Sci. Eng. C 45 (2014) 56–63. [24] T. Zhu, C. Li, W. Yang, X. Zhao, X. Wang, C. Tang, B. Mi, Z. Gao, W. Huang, W. Deng, Electrospray dense suspensions of TiO2 nanoparticles for dye sensitized 630