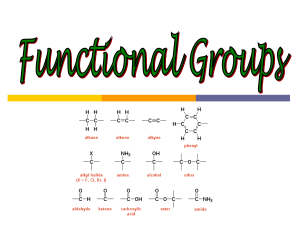
Marine Geochemistry: Ocean Chemistry & Carbon Cycle
Anuncio
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/338051786 Marine Geochemistry Presentation · December 2019 CITATIONS READS 0 18 1 author: Osama Rahil University of Benghazi 213 PUBLICATIONS 4,127 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Project 8: Research, books, articles and achievements of participants in Researchgate Network (researchgate.net) ﻣﻨﺸﻮرات اﻟﺰﻣﻼء اﻟﻤﺸﺘﺮﻛﻴﻦ ﻓﻲView project Geochemistry and Geochronology of the Exposed Rocks in the Cyrenaica Basin, NE Libya View project All content following this page was uploaded by Osama Rahil on 19 December 2019. The user has requested enhancement of the downloaded file. LECTURES FOR UNDERGRADUATE STUDENTS MARINE GEOCHEMISTRY Dr. Osama Shaltami Department of Earth Sciences Faculty of Science, Benghazi University, Libya Ocean Chemistry Figure 4.17a http://www.nhptv.org/ Distribution of Earth’s Water • • • • • • Oceans Ice Caps and Glaciers Atmosphere Rivers and Lakes Inland Seas Groundwater 97.2 % 2.15 % 0.001 % 0.009 % 0.008 % 0.625 % Ocean Water is SALTY • Salinity: Total amount of dissolved solids • Units: o/oo = 1/1000 • Range: 33 – 37 o/oo • Increase in salinity: – – – – Increase in Density Decrease in Freezing Point Decrease in Vapor Pressure Increase in Osmotic Pressure Origin of Salts in Oceans • Rivers (largest transport of chemicals to ocean) – Rain + CO2 H2CO3 – Si, Al, Na, K, Mg • Volcanoes – Cl, S, CO2 • Dust / Rain – Fe, Si • Anthropogenic – CO2, P Example Geochemical Cycle Concept of Steady State Example 2 Geochemical Cycle Residence Time (T = Ocean amount/Output rate) • Concentration of elements in seawater is determined by their removal rate • Conservative elements: – Major Elements: Cl, Na, SO4, Mg, Ca, K - Minor Elements: Br, Sr, B, C, F • Non Conservative Elements – Nutrients: N, P, Si – Dissolved gases: O2, CO2, N2 – Trace Elements: Fe, Al, Mn – Organic Compounds Residence Time - Concentration Element Res. Time (yrs) Concentration Crust (%) Ocean (mg/l) Na Cl Mg K SO4 Ca Mn Fe 60 000 000 80 000 000 10 000 000 6 000 000 9 000 000 1 000 000 7 000 100 2.4 0.013 2.3 2.1 0.026 4.1 0.5 2.4 10 770 19 500 1 290 380 905 412 0.0002 0.002 Dissolved Gases Gas Solubility: Decreases with Temp. and Salinity Increases with Pressure Gases in Atmosphere & Oceans Percent Gas Phase by Volume Gas Atmosphere Surface Ocean Total Ocean N2 79% 48% 11% O2 21% 36% 6% CO2 0.04% 15% 83% Seawater pH • Pure water pH = 7 • Seawater pH = 7.5 – 8.1 • Seawater is very well buffered! CO2(gas)+H2OH2CO3H++HCO32H++CO32 H2O: Universal Polar Solvent H20: Temperature and Density H2O: Frozen & Liquid density H2O: Heat Capacity • Heat Capacity: heat needed to change the temperature of a substance • Water has higher heat capacity than: – All solids – All liquids, except liquid ammonia • Latent heat of Vaporization: heat needed to evaporate a liquid – Water has the highest of all substances Seawater: Temperature and Density Seawater: Temperature and salinity Seawater: Ice Formation Electromagnetic wave penetration Open water (low productivity) Coastal and Estuarine waters (high productivity) Nuclear Missile Submarine How do we measure light penetration? Secchi Disc Water Refraction Eschrichtius robustus Sound Velocity • Influenced by Salinity, Temperature and Pressure – Increases with Salinity – Increases with Temperature – Increases with Pressure • Concept of Midwater Sound Channel Study in Detail Fig 5-19! Sound Channel Humpback Whales Megaptera novaeangliae Gray Whale & Sonar Proposal Eschrichtius robustus Gray Whale Migration Acoustic Pollution The Organic Carbon Cycle Divided into two parts : 1. Biological cycle 2. Geological cycle Biological cycle Photosynthesis in surface waters of oceans or lakes – organic matter from carbon dioxide – organic matter from bicarbonate Ends with metabolic or chemical oxidation of decayed biomass to carbon dioxide Geological cycle • Incorporation of biogenic organic matter into sediments and soils • Leads to the formation of natural gas, petroleum and coal or metamorphic forms of carbon Organic matter accumulation in sediments • In the fossil record: – Dark colored sediments • periods of time favorable to organic matter accumulation – White or red colored sediments or rocks • devoid of organic matter Causes leading to deposition of massive organic-matter rocks • Good Preservation – Sluggish circulation in the deep ocean – Shallow epicontinental seas accompained by water column stratification • Good Productivity – High primary productivity in a dynamic system Primary Production Photosynthetic plankton – produce 20 to 30 billions tons/year of carbon – fixation is not evenly distributed on the oceans but display zones of: • Higher activity on continental margins • Lower activity within the central ocean gyres Export to the Ocean Bottom • Of the total biomass formed only a very small portion reaches the underlying sea floor and is ultimately buried a sediment • Most of the organic matter enters the biological food web and it is respired or used for new biomass production Sedimentation Rate vs. Organic Matter Burial • Oxic open-ocean conditions: – 2X increase in organic carbon content for every 10X increase in sedimentation rate in marine sediments • Anoxic conditions: – no change in organic carbon content over a wide range of sedimentation rates Organic Carbon Content of Marine Sediments • Mean organic carbon content : – 0.3% with a median value of 0.1% – (data from deep sea drilling) • Varies over several hundreds of magnitude Organic Carbon Content of Marine Sediments Depends on: – extend of supply of organic matter – preservation conditions – dilution by mineral matter Chemical Composition of Biomass • Chemical nature of biomass is commonly described by its elemental composition • Marine phytoplankton – Redfield et al. (1963) ratio C:N:P = 106:16:1 • Ratio changes drastically : – food chain processes – early digenetic processes Chemical Composition of Biomass • Chemical composition can also be confined to a limited number of compound classes • Their proportions will vary in the different groups of organisms (Romankevitch, 1984) Principle of Selective Preservation Organic compounds and compound classes: • differ in their potential to be preserved in sediments • differ in their potential survive early diagenesis Principle of Selective Preservation Low Preservation Potential = easily hydrolyzed – Water-soluble organic compounds – Organic macromolecules High Preservation Potential = low solubility in water – Lipids – Hydrolysis resistant molecules Biological Markers • Molecules with high degree of structural complexity provide the possibility of relating a certain product to a specific precursor • EXAMPLE: – 24-methylenecholesterol and dinoserol are preferentially biosynthesized by diatoms and dinoflagellates (Volkman et al., 1998) Marine vs. Terrigenous Organic Matter Variations in marine and terrigenous organic matter proportions important for: – paleoclimatic studies – paleoceanographic studies Parameters used to assess the organic matter sources • Carbon / Nitrogen Ratio – 10 in marine / 20 in terrigenous • Hydrogen Indices (mg HC/g TOC) – 150 in marine / 300-800 in terrigenous • Stable Carbon Isotope Rations d13C = -27o/oo in marine / - 7o/oo in terrigenous Molecular Paleo-Seawater Temperature and Climate Indicators • Biosynthesis of Long-Chain Alkenones in the microalgae Class Haptophyceae depends on the water temperature during growth •Coccolithoophorids belong to this class ! Thanks View publication stats