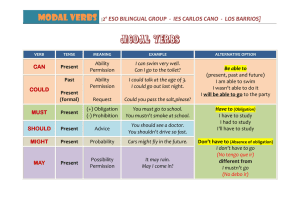
Progress in mineralogical quantitative analysis of rock samples application
Anuncio

TECHNICAL ARTICLE Progress in mineralogical quantitative analysis of rock samples: application to quartzites from Denali National Park, Alaska Range (USA) Mă rgă rit M. Nistor,1 Nicolae Har,2 Simona Marchetti Dori,1 Simona Bigi,1 and Alessandro F. Gualtieri1 1 Dipartimento di Scienze Chimiche e Geologiche, Università di Modena e Reggio Emilia, Via S. Eufemia 19, I-41121 Modena, Italy 2 Department of Geology, University of Babeş-Bolyai, Kogălniceanu Street 1, 400084 Cluj-Napoca, Romania (Received 9 September 2015; accepted 29 October 2015) This work deals with the determination of the mineralogical composition of three quartzite samples, selected as case study to verify the viability and accuracy of various experimental techniques commonly used in geometallurgy and petrography for the determination of the mineralogical composition of rock samples. The investigated samples are from the North-Eastern side of the Denali National Park (Alaska Range, USA). The mineralogical phase abundance of the samples was determined by digitally assisted optical modal point counting, scanning electron microscopy (SEM) + energy dispersive spectroscopy (EDS) modal and digital image analysis, normative calculation from bulk chemistry calculation, and modal Rietveld X-ray powder diffraction. The results of our study indicate that the results provided by modal optical and SEM digitalized counting seem less accurate than the others. The determination with EDS mapping was found to be inaccurate only for one sample. Agreement was found between the X-ray diffraction estimates and bulk chemistry calculation. For both modal optical and SEM digitalized counting, the statistics was probably insufficient to provide accurate results. The estimates obtained from the various methods are compared with each other in the attempt to attain general indications on the precision, accuracy, advantages/disadvantages of each method. © 2016 International Centre for Diffraction Data. [doi:10.1017/S0885715615000871] Key words: Rietveld method, Denali quartzite, optical counting, SEM, EDS, bulk chemistry calculation I. INTRODUCTION The samples investigated in this study are from the North-Eastern side of the Denali National Park (Alaska Range, USA). The Alaska Range represents a tectonically active zone, where Pacific Plate is subducted under North America Plate. The study area is located in the central part of Alaska, and precisely in the external curvature of Alaska Range. The entire region of Central and South Alaska is crossed by a complicated system of faults and thrusts (Csejtey et al., 1982; Singh et al., 2007). The most important is the Denali Faults Systems whose length is around 2000 km long (Hickman et al., 1978). The occurrence of the samples used for the present investigation is from the North-Eastern side of Denali National Park. Denali National Park occupies a large sector of Alaska Range, including Mount McKinley and its surroundings. This park is the oldest preserved area in Alaska (about 24 500 km2) as it was instituted in 1917. The investigated area (see Figure 1) included the Southern part of Mount Margaret and Savage River (between 63°30′ –63°50′ N and 149°00′ –149°30′ W). Savage River is characterized by a large valley, modelled by glaciers in Pleistocene and forms the canyon between Mount Margaret and Mount Healy. The geology of the area shows metamorphic and magmatic complexes alternated with sedimentary rocks. The low lands and valleys are covered by gravels and unconsolidated deposits. Overall, Mount Margaret is constituted by sedimentary rocks dating from Cambrian to Devonian, with a great variety 31 Powder Diffraction 31 (1), March 2016 of rock types. Under the microscope, collected samples 602, 607, and 608 represent sericite-rich quartzites. These rocks display quartz as major component and have inhomogeneous structure with porphyry texture. For the present study, we have focused exclusively on the quartzite rocks with the aim of presenting an accurate mineralogical characterization of these samples. This investigation is preparatory for a more general project in progress about the petrology and petrogenesis of these rock-types which is part of the PhD thesis of the first author M. N. M. Mineralogical quantitative phase determination of rock samples is a method used since more than 60 years in mineralogy, petrography, geometallurgy, archeometry, materials science, and other scientific applicative disciplines. Until the end of the last century, the mineralogical phase composition or phase abundance (modal composition) was typically determined by three methods: (i) point-counting techniques using optical microscopy (optical modal analysis). The optical mode is determined by direct observation and can be assigned a measure of precision (Chayes, 1956). Generally, this method is inappropriate for fine-grained and/or very altered rocks with a grain size usually lower than 10 µm. Besides that, determination suffers from rock heterogeneity, some components can be misidentified, and errors can arise from the conversion from two-dimensional to three-dimensional data; 0885-7156/2016/31(1)/31/9/$18.00 © 2016 JCPDS-ICDD 31 Figure 1. (Colour online) Location of the samples on the Alaska map and Margaret Mount surroundings. Maps source: UAF-GINA/SDMI http://alaskamapped. org/bdl. (ii) normative calculation from bulk chemical data (Barth, 1959). Norms are theoretical mineral abundances calculated from a bulk chemical analysis. This technique is chosen in place of optical microscopy for fine-grained rock samples although not all rock types are adequately modelled by the standard calculation procedures and, because ideal compositions are assumed for the minerals, small variations in bulk chemistry that can strongly affect the resultant mode (Hill et al., 1993). In general, it is not expected that norms will match the mode, except for simple rock compositions. The use of multicomponent chemical mass balancing in solving mineral quantities from chemical analysis [e.g. X-ray fluorescence (XRF)] has been successfully used for decades by a number of researchers and laboratories (see for example Lamberg, 2011; Lund et al., 2013). Normative calculations used by geologists for classification purposes, for example Cross, Iddings, Pirsson, and Washington (CIPW) (Cross et al., 1902), differ from mass balancing method by calculating hypothetical and not actual mineral composition for samples, using stoichiometric compositions for minerals (e.g. CIPW uses volatile free minerals) and reporting mineral grades as normative per cent, not as weight proportions. In this paper, we will refer to this method as “bulk chemistry calculations”; (iii) modal X-ray powder diffraction (XRPD) (including the Rietveld method: Hill et al., 1993). The Rietveld method has been used to obtain reliable modal quantitative phase analysis (QPA) estimates (Rietveld, 1969; Hill and Howard, 1987; Bish and Howard, 1988; Bish and Post, 1993; Madsen and Scarlett, 2008) since about 25 years. In this method, the mass fraction (wi) of each ith 32 Powder Diffr., Vol. 31, No. 1, March 2016 crystalline component in a mixture can be calculated from the following equation: w S (Z M V ) i = i i i i wj Sj (Zj Mj Vj ) j (1) j where Si is the refined scale factor, Zi is the number of formula units in the unit cell, Mi is the mass of the unit formula and Vi is the elementary cell volume. QPA using the Rietveld method relies upon the normalization condition (Σi wi = 1). In the last 15 years, a significant progress has been made in this field because of both the advent of image digitalization and analysis and the application of electron microscopy techniques. Image analysis with optical microscopy makes the modal method faster but still positive identification of minerals is not always easy especially if a process sample comprised of very fine-grained particles. Scanning electron microscopy (SEM)-based image analysis (Quantitative Evaluation of Minerals by SCANning electron microscopy (QEMSCAN®), mineral liberation analyzer (MLA), pressure transient analysis (PTA), IncaMineral) provides more rapid modal quantitative analysis of mineral grades and textures (Gu, 2003; Moen, 2006; Liipo et al., 2012). The drawbacks of the method are: (i) it is standardless; (ii) for accurate analysis from particulate materials, the sample should have a narrow size distribution to avoid classification in sample preparation and to eliminate the need for changing magnification during image collection (Kwitko-Ribeiro, 2011); there are limitations on the particle size. For sound statistics one needs to analyse thousands of particles; for very fine particle and mineral grain sizes (<5 µm), the Mă rgă rit M. Nistor et al. 32 resolution starts to be a problem in mineral identification as the electron beam diameter approaches the size of individual grains; minerals with very similar chemical composition are difficult to identify in SEM-based image analysis (Lund et al., 2013). For these reasons this method is widely used in geometallurgy but less in other research fields. In this work, we have selected three quartzite samples from Denali National Park as case study to apply the various experimental techniques from geometallurgy and petrography used for the determination of the mineralogical quantitative analysis of rock samples. It was not possible to collect more than three samples during the field expedition because of the hard environmental conditions. Hence, this work should be considered a preliminary methodological investigation which calls for additional investigations using a larger statistically meaningful number of samples. The mineralogical phase abundance of the samples has been calculated with modal optical microscopy and digital image analysis, modal SEM + energy dispersive spectroscopy (EDS) and digital image analysis, XRF bulk chemistry calculation, and modal Rietveld QPA. Results from the various methods are compared with each other and general indications on the precision, accuracy, advantages/disadvantages of each method are outlined. 607 and 608) the first terrace and the left bank of river, that stretch between 760 and 800 m. Samples 607 and 608 represent sericite-rich schists and quartzites (Figure 1). In the laboratory, raw samples were quartered to obtain about 10 g of homogeneous representative material and subsequently dried at 105 °C for 2 h. B. Digitally assisted optical point counting An optical microscopy study of samples 602, 607, and 608, aimed at determining the modal composition was conducted on thin sections obtained from selected representative fragments. Using polarized light microscopy and a camera, representative images at 50× were obtained. Visual observation was supported by statistical analysis on three selected pictures for each sample using the ImageJ open source software (version 1.42q, Wayne Rasband National Institute of Health, USA: Schneider et al., 2012). Each observation was conducted with the automatic particle analyse procedure available in the software. The obtained figures allowed determination of an independent mineralogical determination of the crystalline components. C. SEM/EDS II. EXPERIMENTAL A. Sample collection A representative amount of each sample (around 2 kg) was collected from the southern slope of Mount Margaret and from Savage River Valley, upstream of the Savage Canyon. The outcrops of Mount Margaret show the presence of igneous and metamorphic rocks. Sample 602 is from Mount Margaret (Figure 1) composed of gabbroic rocks, quartzites, and schists. The specimens were sampled at 1000–1200 m altitude. Even if the bottom of Savage Valley is constituted by rewashed gravels deposited in the river suspension, the characteristics of samples taken from Savage Valley (Figure 1) do not differ greatly from the samples from Mount Margaret. In Savage Valley we have outcropped (samples TABLE I. Results of the mineralogical quantitative determination of the investigated samples. Phase (wt%) Sample 602 Chlorite Mica Quartz Others Sample 607 Chlorite Mica Quartz Others Sample 608 Chlorite Micac Quartz Others The modal quantitative determinations using the combined SEM/EDS analysis was conducted using a FEI Quanta 200 environmental scanning electron microscopy (ESEM) instrument equipped with an energy dispersive XRF X-EDS Oxford INCA-350 spectrometer. For the observations, the thin sections of samples 602, 607, and 608 used for the optical microscopy were loaded on aluminium stubs. Measurements were performed in low vacuum mode without coating. Micrographs of each sample were collected in triplicate at 500 and 1000× magnifications using back-scattered electrons. The obtained images were digitalized and analysed through using both ArcGIS ESRI version 10.1 and ImageJ open source version 1.42q (Wayne Rasband National Institute of Health, USA) software to determine the phase fraction of each mineral phase on the basis of the crystal habit/morphology. In ArcGIS, QPA Rietvelda Optical counting EDS mappingb Chemistry bulk calculation 0.7(2) 13.7(2) 85.6(1) – – 10.1 89.9 – – 5.4 89.6 5.0 – 13.0 84.6 2.4 2.5 14.1 83.4 – – 6.7(2) 93.3(2) – – 9.0 91.0 – – 11.3 88.3 0.4 – 11.3 88.7 – 1.1 4.3 94.6 – 2.3(2) 11.7(1) 86.0(1) – – 9.6 90.4 – – 13.5 86.2 0.3 – 13.5 83.9 2.6 4.7 10.0 85.3 – SEM The agreement factors of the refinements are: 602 Rwp = 15.33%, Rp = 12.22%, and χ2 = 7.723; 607 Rwp = 16.11%, Rp = 12.18%, and χ2 = 12.0; 608 Rwp = 15.67%, Rp = 11.63%, and χ2 = 11.53. b Merge of both 500 and 1000×. c The term mica includes both muscovite and biotite. a 33 Powder Diffr., Vol. 31, No. 1, March 2016 Quantitative analysis of quartzites from Alaska Range (USA) 33 mineral phase. To do that, morphological inputs and data of the chemical spot analysis were used. The fraction of each coloured area was calculated using ImageJ by automatic decomposition of each RGB channel and using the particle analyse function. With this procedure, it was possible to accomplish the fraction of each coloured fraction of each image and the final composition was obtained by merging all the single image data. The mineralogical quantitative determination was also attempted using an automatic elaboration of elemental X-EDS distribution maps for samples 602 and 607. The software ImageJ was used again for the data analysis. In the first step, the K-map and Al-maps were subtracted from the Si-map so as to enhance the areas where Si concentrates (white areas, related to quartz) with respect to the areas where the other elements such as K, Al, and Fe concentrate (dark areas corresponding to mica and accessory minerals). The next step was to apply a 6–10 pixel wide filter to render the image more homogeneous. Contrast and lightness were optimized to isolate the white areas form the dark areas. The obtained image was digitalized to binary format and analysed with the tool particle analyse. Figure 2. SEM image and relative EDS scan of a well-defined crystal of presumably goethite found inside sample 602. the high capacity of raster data analysis, allowed us to quantify the minerals in two ways: manual vectorization applied to each mineral phase and histogram analysis. Prior to the analysis using ImageJ, the images were digitalized using the free vector graphics editor Inkscape version 0.48.5 and images were decomposed into different colours related to each observed D. XRF XRF data of the major oxides on samples 602, 607, and 608 for the bulk chemistry calculation were collected using a Philips PW 1480 spectrometer. The content of the structure volatiles was independently determined by Thermogravimetric (TG analysis) using a SEIKO SSC 5200 instrument in the temperature range 20–1100 °C and a heating rate of 10 °Cmin−1. Given the very simple mineralogical assemblage, the Figure 3. (Colour online) Selection of optical micrographs. Legend: (a) section of 602 sample (10×); (b) section of 607 sample (10×); (c) section of 608 sample (10×); and (d) section of 602 sample (10×) showing an example of the digitalization procedure of the mineral selection using ArcGIS. 34 Powder Diffr., Vol. 31, No. 1, March 2016 Mă rgă rit M. Nistor et al. 34 Figure 4. (Colour online) Gallery of SEM micrographs. Legend: (a) sample 602, 500× (the marker is 300 µm wide); (b) sample 607, 500× (the marker is 300 µm wide); (c) sample 608, 500× (the marker is 300 µm wide); (d) sample 602, 1000× (the marker is 300 µm wide); (e) sample 607, 1000× (the marker is 300 µm wide); (f) sample 608, 500× (the marker is 300 µm wide); (g) section of 602 sample (500×) showing an example of the digitalization procedure of the mineral selection using ArcGIS. calculation of the mica muscovite weight fraction was determined as: wt% muscovite = (determined wt%K2O × 100)/ 10.01. The value of 10.01 corresponding to the wt% K2O of pure muscovite [KAl2(OH)2Si3AlO10], was calculated from experimental data (the average of 23 chemical compositions of muscovite samples from Volk, 1939). In turn, all MgO wt % has been assigned to ideal clinochlore [Mg5Al (OH)8Si3AlO10] (Brown and Bailey, 1963). The difference with respect to 100 wt% is the wt% fraction of quartz. Fragments of the original rock samples were manually crushed and pre-ground using an agate mortar. The rather coarse material was dry-milled for 20 min using an agate mill. Final milling was performed for 40 min using a micromill with SiC balls as grinding media at low speed, to produce a fine powder but not to overgrind it with the risk to produce amorphous silica or amorphous phases (this procedure has been tested in our laboratory using standard sample powders). XRPD data for both qualitative and quantitative analyses were collected using a Bragg–Brentano θ–2θ diffractometer (Philips PW 1710 with PW 1729 goniometer, PANalytical, The Netherlands) equipped with a gas proportional detector. The experimental set-up accomplished: a ½° divergence slit, 0.04 rad soller slits, and a ½° anti-scatter slit on the incident beam pathway, a 0.2 mm receiving slit, 0.04 rad soller slits and a curved graphite monochromator on the diffracted beam. The radiation source was an X-ray tube with copper radiation (λCuKα1,Kα2 = 1.540 59, 1.544 39 Å) and the anode tube load was 40 kV and 30 mA. The samples were sideloaded on a quartz flat zero background holder. XRPD patterns were collected at room temperature in the 5°–80°2θ range, with a scanning rate of 0.004°s−1 and a step size of 0.02°2θ. Preliminary qualitative phase analyses were performed using the X’Pert High Score Plus software (PANalytical, The Netherlands). Rietveld QPAs were performed using the GSAS package (Larson and Von Dreele, 1999) and its graphical interface EXPGUI (Toby, 2000). The starting atomic coordinates for all phases in the investigated systems were taken from the literature: chlorite (clinochlore: Zanazzi et al., 2009), muscovite (Guggenheim et al., 1987), and quartz (Gualtieri, 2000). The background profile was fitted with a Chebyshev polynomial function with 8–12 coefficients. The profile of the diffraction peaks was modelled using a 35 Quantitative analysis of quartzites from Alaska Range (USA) E. Rietveld QPA Powder Diffr., Vol. 31, No. 1, March 2016 35 Figure 5. An example of back-scattered electrons image of sample 602 (a) and the relative elemental distribution map of silicon (b), and iron (c). The map digitalization using an automated procedure using the ImageJ software is shown for silicon (d), and for iron (e). pseudo-Voigt function with one Gaussian and two Lorentzian coefficients in addition to an instrumental peak asymmetry component set to be equal for all phases. III. RESULTS AND DISCUSSION Table I reports the mineralogical quantitative determination from different methods for the three investigated samples 602, 607, and 608. Quartz is obviously the major phase with subordinate mica (muscovite), and minor chlorite in 602 and 608. The SEM investigation also revealed traces of other impurities such as amorphous of poorly-crystalline iron oxides/ hydroxides, present as alteration products (Figure 2 with the image of a well-defined crystal and its EDS chemical composition), rutile and zircon. Such impurities are below the detection limit of X-ray diffraction. Figure 3 reports a gallery of optical images for the investigated samples 602 (a), 607 (b), and 608 (c) at 10× magnification. For sample 602 at 10× magnification, Figure 3(d) shows an example of the digitalization procedure of the mineral selection using ArcGIS (ESRI, 2011). Figure 4 is a selection of SEM micrographs for the investigated samples 602 at 500× magnification (a), sample 607 at 500× magnification (b), sample 608 at 500× magnification (c), sample 602 at 1000× magnification (d), sample 607 at 1000× magnification (e), and sample 608 at 1000× magnification (f). For 602 sample (500×), an example of the image digitalization procedure of the mineral selection using ArcGIS is portrayed in Figure 4(g). 36 Powder Diffr., Vol. 31, No. 1, March 2016 Table I reports the results of the determinations for the three samples using the various experimental techniques. The first column reports the results of the XRPD QPA using the Rietveld method. The second column reports the software assisted counting of grains of different mineral species from optical microscopy in transmission mode. The third column reports the software assisted counting of grains of different mineral species from SEM imaging. Results from the analysis of images at 500 and 1000× magnification, and using two different programs (ArcGIS and ImageJ) were merged. Insignificant differences were detected with respect to the magnification and software used. The fourth column is relative to the results obtained from the digitalization of the EDS elemental maps using ImageJ. Figure 5 reports an example of back-scattered electrons image of sample 602 (a) and the relative elemental distribution map of silicon (b) and iron (c). The map digitalization using an automated procedure in ImageJ is shown in Figure 5(d) for silicon and (e) for iron. The last column of Table I reports the phase fractions calculated from the bulk XRF chemical analysis. Table I reports the Rietveld quantitative phase composition of the samples with the relative agreement factors (as defined in Larson and Von Dreele, 1999). The Rietveld graphical outputs are shown in Figures 6(a–c). Figures 7(a–c) are histogram plots for the three samples of the fractions of quartz, mica, and other accessory minerals (chlorite) determined with the various experimental techniques. The horizontal full line is the calculated mean and the broken lines are relative to the standard deviation. With the aid of the standard deviation, the numbers which greatly depart from the mean value can be appreciated. Mă rgă rit M. Nistor et al. 36 Figure 6. (Colour online) The Rietveld graphical outputs for sample 602 (a), 607 (b), and 608 (c). Markers relative to the reflections of the crystalline phases present in the systems in the selected 2θ range are also shown together with the difference curve between the observed and calculated pattern. In Figure 7(a), the mean value for quartz is 86.6 (2.6) wt%. Both EDS mapping and bulk chemistry calculation are in line with the results from the Rietveld method. On the other hand, both optical and SEM counting appear to be overestimated. The same applies to mica. The mean value for mica is 11.3 (3.4) wt%. The quantitative determination with the Rietveld method shows results similar to the EDS mapping and bulk chemistry calculation. They are all close to the mean value [Figure 7(a)]. The optical counting is instead underestimated and the SEM counting is grossly underestimated. This may indicate that the statistics used of the SEM method was probably not satisfactory and that during the measurements, areas with abundance of quartz grains with respect to mica were presumably observed. For sample 607, Figure 7(b) shows that the mean value for quartz is 91.2 (3.1) wt%. The modal optical counting and bulk chemistry calculation are close to the values from the Rietveld method and also close to the mean value. Both modal SEM 37 Powder Diffr., Vol. 31, No. 1, March 2016 Figure 7. Histogram plots showing the fractions of quartz, mica, and chlorite (others) determined with the various experimental techniques. The horizontal full line is the calculated mean and the broken lines are relative to the standard deviation. Legend: (a) sample 602; (b) sample 607; and (c) sample 608. counting and EDS mapping are underestimated although rather close to the standard deviation. The same applies to the results observed for muscovite. The mean value for mica muscovite is 8.5 (3.1) wt%. Although the quantitative determination of all the methods except the bulk chemistry calculation is close to the mean value [Figure 7(b)], SEM and EDS mapping determinations are fairly different from the results of the Rietveld method. The determination of the other phases is statistically not significant. In Figure 7(c) (sample 608), the mean value for quartz is 86.2 (2.5) wt%. The quantitative determinations are all within the limits of the standard deviation. SEM and bulk chemistry calculation convey results close to those determined with the Rietveld method. Instead, the determination with the modal optical counting is instead strikingly overestimated. The Quantitative analysis of quartzites from Alaska Range (USA) 37 mean value for mica is 11.7 (2.3) wt%. All the quantitative determinations are within the limits of the standard deviation and close to the results from the Rietveld method. The few observations for the other phases prevent any statistically significant consideration. In the section Introduction, we have reported that it was not possible to collect more than three samples during the field expedition because of the hard environmental conditions and that this work should be considered a preliminary methodological investigation. Notwithstanding, despite the small number of observations (samples), it is possible to highlight that the results which apparently show major scatter and larger departure from the Rietveld estimates are from modal optical and SEM counting. Good agreement is found between the X-ray diffraction estimates and bulk chemistry calculation as they are both bulk methods with high statistics significance. Although we have analysed a number of sections for both the optical and SEM digitalized counting, the statistics is probably insufficient to provide accurate results comparable with those accomplished with bulk methods. A protocol of analysis should be drawn by defining the minimum number of fields to be analysed for both modal optical and SEM counting to obtain statistically meaningful accurate data. Our findings are in line with those reported for the case of heavy minerals in sandstones for which Webster et al. (2003) reported that the optical/SEM area observations should be considered preliminary especially for epidote, augite, feldspars, and talc. More confidence could be placed in the optical-SEM/EDS results if they included microanalysis of all questionable grains present in each of the three size fractions. Although Webster et al. (2003) claimed that there is reasonable agreement between mineral abundances determined from the optical/SEM work and Rietveld refinements, significant difference between the two methods was found in the paper concerning the abundance of the dominant phase diopside: Rietveld refinements yielded 46 wt% whereas optical/ SEM work yielded 56–57 wt%. This difference was probably greater than uncertainty in optical identifications or the limited number of refinements can explain and was likely because of the effects of preferred orientation not properly accounted for in the Rietveld refinements. On the same line, such discrepancies have also been recently observed in a different more complex system, e.g. cement–asbestos (Croce et al., 2014). There is certainly a dependence of the accuracy of the results upon the nature of the sample as other authors (Gonzalez et al., 2003) reported that despite uncertainties, the results of the Rietveld QPA of volcanic rocks (dacite) compare quite well with optical/SEM results. Le Saoût et al. (2011) observed a good agreement between SEM and Rietveld data as far as the alite/belite ratio in anhydrous cements is concerned. It should be remarked that the accuracy of the estimates from bulk chemistry calculation are favoured by the simple mineralogical assemblage displayed by our samples but cases of more complex systems such as ultramafic rocks which display accurate results are also reported in the literature (Hill et al., 1993). As a matter of fact, determinations with bulk chemistry calculation can be affected by large discrepancies as the chemical composition of the mineral phases in the system is not well defined. If the choice of the chemical composition is correct, it is found that model-derived bulk analysis and optical modes may display comparable results. Although normative mineralogy is based on complete equilibrium 38 Powder Diffr., Vol. 31, No. 1, March 2016 crystallization and may not exactly represent the volumetric proportions in the mode, it at least provides a basis for comparison, and indicates that the optical modes are reasonable (McBirney, 1993; Hamilton and Christensen 2000). IV. CONCLUSIONS In the present work, three quartzite samples from Alaska were selected as case study to test the various experimental techniques commonly used for the determination of the mineralogical quantitative analysis of rock samples (modal optical counting, modal SEM + EDS counting, bulk chemistry calculation, and Rietveld QPA). The findings of this work may be of help to select the best non Rietveld method to perform quantitative-/semi-QPA on natural (rock) or industrial samples such as cements and clinkers. Despite the limited relevance of our statistical analysis, it was found that the less accurate results are apparently obtained from modal optical and SEM digitalized counting. The determination with EDS mapping was found to be inaccurate only for sample 607. Agreement was found between Rietveld QPA estimates and bulk chemistry calculation. For both optical and SEM digitalized counting, the statistics was probably insufficient to provide accurate results comparable with those accomplished with bulk methods. A protocol of analysis should be outlined by defining the minimum number of fields to be analysed for both optical and SEM counting to obtain statistically meaningful accurate data, accepting a compromise between accuracy and time of analysis. The analytical plan used hereby should be applied to more complex natural and synthetic systems in the attempt to generalize the findings of this work and assess the precision and accuracy of each experimental method even when biasing factors such as the presence of amorphous phase. ACKNOWLEDGEMENTS G. Urso of the Centro Interdipartimentale Grandi Strumenti (CIGS) of the University of Modena and Reggio Emilia is kindly acknowledged for help with the SEM analyses. SUPPLEMENTARY MATERIALS To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0885715615000871 Barth, T. F. W. (1959). “Principles of classification and norm calculations of metamorphic rocks,” J. Geol. 67(2), 135–152. Bish, D. L. and Howard, S. A. (1988). “Quantitative phase analysis using the Rietveld method,” J. Appl. Crystallogr. 21, 86–91. Bish, D. L. and Post, J. E. (1993). “Quantitative mineralogical analysis using the Rietveld full-pattern fitting method,” Am. Mineral. 78(9–10), 932–940. Brown, B. E. and Bailey, S. W. (1963). “Chlorite polytypism: II. Crystal structure of a one-layer Cr-chlorite,” Am. Mineral. 48, 42–61. Chayes, F. (1956). Petrographic Modal Analysis: an Elementary Statistical Appraisal (Wiley, New York), p. 113. Csejtey, B. Jr., Cox, D. P., Evarts, R. C., Stricker, G. D., and Foster, H. L. (1982). “The Cenozoic Denali Fault System and the Cretaceous accretionary development of southern Alaska,” J. Geophys. Res. 87(B5), 3741–3754. Cross, W., Iddings, J. P., Pirsson, L. V., and Washington, H. S. (1902). “A quantitative chemicomineralogical classification and nomenclature of igneous rocks,” J. Geol. 6, 555–690. Mă rgă rit M. Nistor et al. 38 Croce, A., Allegrina, M., Trivero, P., Rinaudo, C., Viani, A., Pollastri, S., and Gualtieri, A. F. (2014). “The concept of ‘end of waste’ and recycling of hazardous materials: in depth characterization of the product of thermal transformation of cement-asbestos,” Mineral. Mag. 78(5), 1177–1191. ESRI (2011). ArcGIS Desktop: Release 10 (Environmental Systems Research Institute, Redlands, CA). Gonzalez, R. M., Edwards, T. E., Lorbiecke, T. D., Winburn, R. S., and Webster, J. R. (2003). Analysis of geologic materials using Rietveld quantitative X-ray diffraction,” Adv. X-ray Anal. 46, 204–209. Gu, Y. (2003). “Automated scanning electron microscope based mineral liberation analysis,” J. Miner. Mater. Charact. Eng. 2, 33–41. Gualtieri, A. F. (2000). “Accuracy of XRPD QPA using the combined Rietveld-RIR method,” J. Appl. Crystallogr. 33, 267–278. Guggenheim, S., Chang, Y. H., and Koster van Groos, A. F. (1987). “Muscovite dehydroxylation: High-temperature studies, Sample: T = 20 °C, from Diamond mine, Keystone, South Dakota,” Am. Mineral. 72, 537–550. Hamilton, V. E. and Christensen, P. R. (2000). “Determining the modal mineralogy of mafic and ultramafic igneous rocks using thermal emission spectroscopy,” J. Geophys. Res. 105(E4), 9717–9733. Hickman, R. G., Craddock, C. and Sherwood, K. W. (1978). “The Denali Fault systems and the tectonic development of Southern Alaska,” Tectonophysics 47, 247–273. Hill, R. J. and Howard, C. J. (1987). “Quantitative phase analysis from neutron powder diffraction data using the Rietveld method,” J. Appl. Crystallogr. 20, 467–474. Hill, R. J., Tsambourakis, G., and Madsen, I. C. (1993). “Improved petrological modal analyses from X-ray powder diffraction data by use of the Rietveld Method I. Selected igneous, volcanic, and metamorphic rocks,” J. Petrol. 34(5), 867–900. Kwitko-Ribeiro, R. (2011). “New sample preparation developments to minimize mineral segregation in process mineralogy,” In: Proc., 10th Int. Congress for Applied Mineralogy (ICAM), Trondheim, Norway, 1–5 August, 411–417. Lamberg, P. (2011). “Particles – the bridge between geology and metallurgy,” In: Conf. in Mineral Engineering, Proc., Luleå, Sweden, 8–9 February, 1–16. Larson, A. C. and Von Dreele, R. B. (1999). Report LAUR LANL 86–748 (USA, Los Alamos National Laboratory). Le Saoût, G., Kocaba, V., and Scrivener, K. (2011). “Application of the Rietveld method to the analysis of anhydrous cement,” Cem. Concr. Res. 41, 133–148. Liipo, J., Lang, C., Burgess, S., Otterstrom, H., Person, H., and Lamberg, P. (2012). “Automated mineral liberation analysis using INCAMineral,” In: Process Mineralogy ‘12, Proc., Vineyard Hotel, Cape Town, South Africa, 7–9 November, 1–7. Lund, C., Lamberg, P., and Lindberg, T. (2013). “Practical way to quantify minerals from chemical assays at Malmberget iron ore operations – an important tool for the geometallurgical program,” Miner. Eng. 49, 7–16. Madsen, I. C. and Scarlett, N. V. Y. (2008) “Quantitative phase analysis” Chapter 11 in “Powder diffraction: theory and practice,” RSC Publishing, 298–329. McBirney, A. R. (1993). “Igneous Petrology (Jones and Bartlett, Boston, Mass), 2nd ed., 508 pp. Moen, K. (2006). “Quantitative measurements of mineral microstructure,” Doctoral thesis. Norwegian University of Science and Technology, Trondheim, 194 pp. Rietveld, H. M. (1969). “A profile refinement method for nuclear and magnetic structures,” J. Appl. Crystallogr. 2, 65–71. Schneider, C. A., Rasband, W. S., and Eliceiri, K. W. (2012). “NIH Image to ImageJ. 25 years of image analysis,” Nat. methods 9(7), 671–675. Singh, R. P., Cervone, G., Singh, V. P., and Kafatos, M. (2007). “Generic precursors to coastal earthquakes: inferences from Denali fault earthquake,” Tectonophysics 431, 231–240. Toby, B. H. (2000). “EXPGUI, a graphical user interface for GSAS,” J. Appl. Crystallogr. 34, 201–213. Webster, J. R., Kight, R. P., Winburn, R. S., and Cool, C. A. (2003). “Heavy mineral analysis of sandstones by Rietveld analysis,” Adv. X-ray Anal. 46, 198–203. Volk, G. W. (1939). “Optical and chemical studies of muscovite,” Am. Mineral. 24, 255–266. Zanazzi, P. F., Comodi, P., Nazzareni, S., and Andreozzi, G. B. (2009). “Thermal behaviour of chlorite: an in situ single-crystal and powder diffraction study,” Eur. J. Mineral. 21, 581–589. 39 Quantitative analysis of quartzites from Alaska Range (USA) Powder Diffr., Vol. 31, No. 1, March 2016 39