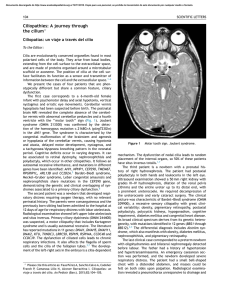
Seminar Idiopathic nephrotic syndrome in children Damien G Noone, Kazumoto Iijima, Rulan Parekh The incidence of idiopathic nephrotic syndrome (NS) is 1∙15–16∙9 per 100 000 children, varying by ethnicity and region. The cause remains unknown but the pathogenesis of idiopathic NS is thought to involve immune dysregulation, systemic circulating factors, or inherited structural abnormalities of the podocyte. Genetic risk is more commonly described among children with steroid-resistant disease. The mainstay of therapy is prednisone for the vast majority of patients who are steroid responsive; however, the disease can run a frequently relapsing course, necessitating the need for alternative immunosuppressive agents. Infection and venous thromboembolism are the main complications of NS with also increased risk of acute kidney injury. Prognosis in terms of long-term kidney outcome overall is excellent for steroid-responsive disease, and steroid resistance is an important determinant of future risk of chronic or end-stage kidney disease. Introduction Nephrotic syndrome (NS) is characterised by the triad of proteinuria, hypoalbuminaemia, and oedema (panel 1). Many glomerular disorders in childhood present with nephrotic syndrome, however, the vast majority are idiopathic NS, and the focus of this Seminar (panel 2). The precise cause of this common childhood disease remains elusive despite substantial advances in our understanding of podocyte biology. Idiopathic NS can be classified on the basis of response to steroid therapy, pattern of relapse, histopathology, or by genetic mutations. Most simply, NS is categorised on the basis of clinical response to steroid therapy, as steroid sensitive (SS) or steroid resistant (SR). Although helpful for guiding therapy, this classification lends very little understanding to disease mechanism. Idiopathic NS is best defined as a podocytopathy due to loss or altered function of the podocytes, resulting in massive proteinuria. The mainstay of treatment for NS is corticosteroids (steroids) with protocols largely based on seminal studies from the International Study of Kidney Disease in Children and the Arbeitsgemeinschaft für Pädiatrische Nephrologie.2,3 Steroid responsiveness and frequency of relapses provide the best guide to therapy in idiopathic NS. The majority of children respond well to steroids within 4 weeks (steroid-sensitive NS [SSNS]); however, most will relapse, with approximately half becoming frequent relapsers or steroid dependent.4,5 Although historically fewer than 10% of children with SSNS continue to have relapses in adulthood,3,6 contemporary cohorts suggest higher proportions of 16∙4–42%. Frequency of relapses during childhood and the need for non-steroid immuno­suppressants such as cyclophosphamide or ciclosporin are predictive of active disease as young adults.7–-10 Among 287 children followed up for over 15 years, 85% achieved long-term remission.11 Despite ongoing relapses, kidney outcomes remain excellent, with risk of progression to chronic kidney disease estimated to be less than 5% in those with SSNS at 10 years after diagnosis.12 In contrast, SRNS is associated with increased risk of progression to end-stage renal disease (ESRD).13 Children with SRNS on biopsy www.thelancet.com Vol 392 July 7, 2018 might have minimal change or focal segmental glomerulosclerosis (FSGS). Owing to the heterogeneity of SRNS, only 50% are at risk of progressing to ESRD in 5 years; typically those children who do not achieve complete or partial remission.14 Long-term prognosis in adults with paediatric onset NS is not well studied and would provide important information on risk for families. Standard definitions are established and highlighted in the 2012 Kidney Disease Initiatives: Global Outcomes (KDIGO; panel 1).1 Clinical presentation Classically, a child presents with a history of progressive oedema, initially periorbital and noticeable in the morning. There can be an antecedent infection, typically of the upper respiratory tract. Urine output is described as frothy or foamy. Abdominal pain is relatively common and, if accompanied by fever, could signify spontaneous bacterial peritonitis. Headache with accompanying neurological signs or irritability should raise the suspicion for cerebral venous sinus thrombosis. Clinical investigations are summarised in panel 3. Lancet 2018; 392: 61–74 Published Online June 14, 2018 http://dx.doi.org/10.1016/ S0140-6736(18)30536-1 This online publication has been corrected. The corrected version first appeared at thelancet.com on July 26, 2018 Department of Paediatrics, University of Toronto, Toronto, ON, Canada (D G Noone MB BCh BAO, Prof R Parekh MD); Division of Nephrology, The Hospital for Sick Children, Toronto, ON, Canada (D G Noone, Prof R Parekh); Department of Pediatrics, Kobe University Graduate School of Medicine, Kobe, Japan (K Iijima MD); Child Health Evaluative Sciences, Research Institute, Hospital for Sick Children, Toronto, ON, Canada (Prof R Parekh); University Health Network, Toronto, ON, Canada (Prof R Parekh); and Dalla Lana School of Public Health, and Health Policy, Management and Evaluation, University of Toronto, Toronto, ON, Canada (R Parekh) Correspondence to: Dr Rulan Parekh, The Hospital for Sick Children, Toronto, ON M5G 1X8, Canada [email protected] Pathology Most children do not get a kidney biopsy at presentation. Historical studies have demonstrated that the most Search strategy and selection criteria We conducted a systematic literature search of published literature using Cochrane, PubMed, Embase, and MEDLINE. Key search terms included “children”, “nephrotic syndrome”, “steroid sensitive nephrotic syndrome”, “steroid resistant nephrotic syndrome” and “focal segmental glomerulosclerosis”. The reference lists of all included papers and review articles were also cross-referenced to identify additional relevant studies. The search was not limited by study design, year of publication, or language but precedence was given where possible to randomised controlled trials and studies from the last 5 years. The initial search was done on Nov 29, 2015 and repeated on Dec 1, 2017 to update with the recent published clinical trials. 61 Seminar Panel 1: Relevant definitions in nephrotic syndrome1 Nephrotic syndrome (NS) Oedema with protein excretion >40 mg/m² per h or urine protein:creatinine ratio ≥2000 mg/g (≥200 mg/mmol) or >3+ proteinuria on dipstick with serum albumin <2∙5 g/dL (25 g/L) Remission Urine albumin trace or negative on dipstick or proteinuria <4 mg/m² per h or urinary protein:creatinine ratio <200 mg/g (20 mg/mmol) for 3 consecutive days Panel 2: Causes of non-idiopathic childhood nephrotic syndrome (NS) • Nephritic/nephrotic glomerular disorders • IgA nephropathy and Henoch–Schonlein purpura • Membranoproliferative glomerulonephritis • Lupus nephritis • Postinfectious glomerulonephritis • Immune complex mediated glomerulopathy • C1q nephropathy • Thin basement membrane disease Relapse Urine albumin 3+ or 4+ or proteinuria >40 mg/m² per h or urinary protein:creatinine ratio >200 mg/g (20 mg/mmol) for 3 consecutive days • Membranous nephropathy Frequently relapsing NS ≥2 relapses within 6 months of initial response or ≥4 in any 12 month period • Interstitial nephritis Steroid-dependent NS 2 consecutive relapses occurring while weaning to alternate day steroids or within 2 weeks of steroid discontinuation Steroid-resistant NS Persistent proteinuria despite 60 mg/m² or 2 mg/kg for 8 weeks, after ensuring no infection or non-adherence to medication common pathological findings in childhood NS are either classified as minimal change and termed minimal change disease (MCD) or FSGS.1 In minimal change, the glomeruli appear normal under light microscopy, with evidence of podocyte effacement by electron microscopy.17 Characteristic histology in FSGS is segmental sclerosis of affected glomeruli, with the segment often adherent to Bowman’s capsule by synechiae.18 • Sickle-cell nephropathy • Thrombotic microangiopathy • Infections associated with NS • Hepatitis B and C • HIV-1 • Malaria • Syphilis • Toxoplasmosis • Varicella zoster • Drugs associated with NS • Non-steroidal anti-inflammatory drugs • Bisphosphonates • d-penicillamine • Heavy metals (mercury and gold) • Lithium • Rifampicin • Sulfasalazine • T-cell-related malignancy • Hodgkin’s lymphoma • Thymoma • Leukaemia Incidence There is considerable variation in incidence of NS depending on country of origin, or ethnicity, with proportions ranging from 1∙15 to 16∙9 per 100 000 children (figure 1).11,19 Incidence is highest in those of south Asian ancestry compared to European ancestry as reported in studies from the UK, South Africa, and Canada. Incidence of steroidresistance ranges from 2∙1 to 27∙3% and also varies by country of origin (figure 1).19 Most studies are retrospective or cross-sectional with only a few longitudinal studies. Reported differences can thus be partially attributable to management variations across practices or regions, as well as use of differing definitions of outcomes. African–American children are more likely to have biopsy-proven FSGS (42–72%)19 and have the highest proportion of progression to ESRD as compared to European Americans,20 whereas 62 in south Asian children, FSGS is reported less commonly and ranges from 15 to 39%.19 Pathophysiology Abnormalities in the podocyte and glomerular filtration barrier The podocyte is a polarised epithelial cell with inter­ digitating foot processes with a unique cell–cell junction known as the slit diaphragm. Along with the glomerular basement membrane and the fenestrated glomerular endothelium, the podocyte forms a trilayered structure— the glomerular filtration barrier. The podocyte and filtration barrier allow an ultrafiltrate almost completely devoid of protein to pass into the Bowman’s space and proceed onto the proximal tubule. Podocyte architecture is maintained by an extensive actin cyto­ skeleton that enables the glomerular filtration barrier to withstand the www.thelancet.com Vol 392 July 7, 2018 Seminar Panel 3: Investigations in a child with nephrotic syndrome (NS) Baseline investigations 1 Urinalysis and urine microscopy 2 Urine albumin or protein:creatinine ratio 3 24-h timed collection of urine for protein quantification 4 Serum electrolytes, albumin, total protein, renal function, and cholesterol A substantial capillary hydrostatic pressure. Loss of normal podocyte structure, the foot processes or the slit diaphragm that spans these interdigitations can lead to loss of albumin in the ultrafiltrate. Podocytes are terminally differentiated cells with minimal regeneration and thus, vulnerable to injury. Complete effacement of the podocyte with the loss of normal architecture results in massive proteinuria, a hallmark of nephrotic syndrome (figure 2). The pathogenesis leading to podocyte effacement is not clear in idiopathic NS, nor the specific mechanism in which treatment with steroids leads to the recovery of podocyte structure and function. Systemic factors, immune mediated or circulating, can contribute to podocyte effacement, but there is no single uni­ fying hypo­ thesis. Supporting evidence from rare or www.thelancet.com Vol 392 July 7, 2018 Multi-ethnic (n= 49)* Australia Not specified (n=135) The Netherlands Not specified (n=231) Kentucky, USA Not specified (n=34) Benghazi, Libya Arabs (n=134) Arabs (n=55) Ohio, USA African-Americans (n=15) Whites (n=157) Kansas City, USA African-Americans (n=25) Europeans (n=54) Erie county, USA Whites (n=9) Non-whites (n=73) Birmingham, UK Afro-Caribbeans (n=2) Europeans (n=15) Asians (n=27) Former Yorkshire, UK Non-Asians (n=49)† Asians (n=121)† Leicestershire, UK Non-Asians (n=22) Asians (n=21) Toronto, Canada East/Southeast Asians (n=69) South Asians (n=237) Europeans (n=173) Others (n= 232) Infectious work-up depending on clinical context 1 Hepatitis B and C, HIV, syphilis, or tuberculosis can also be considered depending on the clinical context Renal biopsy considered in the following situations 1 Age <1 or >12 years 2 Persistent or sustained elevation in creatinine 3 Significant haematuria or gross haematuria16 4 Hypocomplementaemia 5 Findings indicative of another autoimmune disease 6 Infection with hepatitis B or C, HIV, or tuberculosis 7 Hypertension 8 Glucocorticoid resistance Ethnicity (n) New Zealand Farwaniya/Jahra, Kuwait Additional testing if there is a suspicion of a glomerulonephritis 1 Serum complement C3 and C4 concentrations 2 Serum immunoglobulins 3 Antistreptolysin titres 4 Anti-DNAse B antibodies 5 Antinuclear antigen antibodies 6 Anti-double-stranded DNA antibodies 7 Anti-neutrophil cytoplasmic antibodies Consideration of genetic testing 1 A positive family history of NS 2 Congenital NS 3 Infantile onset (<1 year) 4 Failure to respond to steroid therapy 5 Persistent kidney dysfunction 6 Features suggestive of a known syndrome (appendix)15 City, country 0 2 4 6 8 10 12 14 16 18 Incidence‡ (per 100 000 people) B Steroid resistant City, country Ethnicity (total n, SR%)§ New Zealand Multi-ethnic (n=49, 19·6%) Steroid sensitive Durban, South Africa Black and Indian (n=816, 27·3%) Diyarbakir, Turkey Turkish (n=138, 13·2%) Sindh, Pakistan Pakistani (n=538, 31·1%) Siem Reap, Cambodia Cambodian (n=112, 6·2%) Poland Boston and New York, USA Polish (n=178, 24·7%) European (n=65, 6·2%) Black and Hispanic (n=177, 15·3%) New Orleans, USA African-American (n=96, 11·0%) Caucasian (n=103, 3·6%) Toronto, Canada Others (n=232, 2·6%) East/Southeast Asians (n=66, 4·5%) South Asians (n=237, 2·1%) Europeans (n=173, 6·8%) 0 10 20 30 40 50 60 70 80 90 100 % Figure 1: Incidence of childhood nephrotic syndrome per 100 000 persons by ethnicity, reported from 1946 to 2014 (A) and variability of steroid responsiveness by ethnicity among children with nephrotic syndrome in reported studies from 1986 to 2014 (B) Published with permission from Ethnic differences in childhood nephrotic syndrome, published in Frontiers in Pediatrics, 2016.19 n=total number of patients; SR%=proportion of steroid resistance. *New Zealand European, Maori, Pacific Island, Asian, Other. †Only those with steroid-sensitive nephrotic syndrome. ‡Estimated on the basis of data in published studies. familial forms of nephrotic syndrome underscores the importance of genetic variants leading to specific podocyte abnormalities. 63 Seminar Congenital and steroid-resistant forms of NS are associated with mutations in genes encoding com­ ponents of the slit diaphragm, podocyte actin cyto­ skeleton, podocyte mitochondrial proteins, lysosomal proteins, nuclear transcription factors, and glomerular basement membrane (figure 2).21 Maintenance of Figure 2: The glomerular filtration barrier and pathogenesis of idiopathic nephrotic syndrome Within the kidney is the glomerulus, a capillary tuft that filters the blood. The podocyte, glomerular basement membrane and the fenestrated glomerular endothelium comprise the glomerular filtration barrier allowing the ultrafiltrate to enter the urinary space. The podocyte has extensive cellular extensions that interdigitate and these foot processes are connected by the slit diaphragm. In nephrotic syndrome, there is extensive effacement of the podocytes and loss of this barrier to protein, allowing excessive serum albumin to leak into the urine. The pathogenesis of idiopathic nephrotic syndrome is hypothesised to be either immune-mediated, owing to a systemic podocyte-derived circulating factor, or, in rarer or familial forms, a genetic variant. Numerous mutations are associated with steroidresistant nephrotic syndrome that affect various parts of the podocyte itself, or the other constituents of the glomerular basement membrane. These include mutations affecting the podocyte nucleus, mito­chondria or lysosomes, the slit diaphragm or actin cytoskeleton, and the glomerular basement membrane. Nephrin, podocin, and CD2AP, for example, are essential components of a zipper-like structure spanning the interdigitating foot processes of the podocyte, the slit diaphragm and link directly with the podocyte actin cytoskeleton. The actin cytoskeleton is further supported by microfilaments that maintain structural stability and facilitate the dynamic nature of the podocyte structure and function. The importance of these microfilaments is evident as mutations in both α-actinin 4 and INF2, which are involved in actin regulation and polymerisation lead to FSGS. 64 podocyte structure and function is dependent on the molecular interplay between the network of proteins that anchor the podocyte to the glom­ erular basement membrane and maintain its unique structure, and also the crosstalk with the fenestrated glomerular endothelium. Thus, any injury to the Glomerular filtration barrier in nephrotic syndrome Kidney Nephron Glomerulus Glomerular filtrate Afferent capillary Efferent capillary Normal Nephrotic syndrome Pathogenesis (a) Immune-mediated (b) Systemic circulating factors (eg, suPAR) (c) Podocyte related factors (eg, ANGPTL4) (d) Genetic variants Mutant proteins play roles in: 4 Actin 1 Nucleus Urinary space 2 Mitochondria 5 Slit diaphragm 3 Lysosomes 6 Basement membrane Podocyte cell body 1 3 4 2 Slit diaphragm 5 Podocyte effacement Basement membrane 6 Red blood cell White blood cell Fenestrated endothelial cells www.thelancet.com Vol 392 July 7, 2018 Seminar podocyte has implications for the entire glomerular filtration barrier.22 Immune mediated It is suspected that dysfunction or dysregulation of T lymphocytes are involved in the pathogenesis of NS (figure 2).23–25 Supportive evidence includes the efficacy of immunosuppressive agents in NS, spontaneous NS remission following infection with measles,24 and the resolution of NS following chemotherapy for Hodgkin’s and other T-cell lymphomas, which can trigger or precede NS.25 Lastly, development of NS after allergic reactions to various stings and poisons suggests an immunemediated role in disease pathogenesis. A recent molecular candidate for the cause of podocytopathies and proteinuric states is CD80 (B7-1). CD80, is a protein expressed on antigen-presenting cells that provides the primary co-stimulatory signal for T-cell activation via receptors on the T-cell surface. The T-cell surface expresses protein receptor CTLA-4, which binds CD80. An increase in podocyte B7-1 expression is evident in a variety of animal models of proteinuria and in human studies.26 This hypothesis was further tested through the CTLA-4 mimicking therapeutic agents, abatacept and belatacept in FSGS, but remains controversial and clinical trials are under way.27,28 proteinuria, through an effect on either the glomerular basement membrane or the endothelial cells, and by affecting the charge of the glomerular filtration barrier, which prevents albumin from traversing the barrier. In contrast, a sialylated form is released into circulation from other tissues and can mitigate proteinuria by binding αvβ5 integrins on the glomerular endothelium. Exploration of how sialylated ANGPTL4 could be used to treat NS is ongoing, however, off-target effects, such as inhibition of lipoprotein lipase leading to hyper­ triglyceridaemia in NS, could be exacerbated.32 Genetics Genetic risk and SSNS A genetic locus on chromosome 6p and single nucleotide polymorphisms in HLA-DQA1 and HLADQB1 were substantially associated with SSNS using an exome array.36 This locus, however, only explains 4∙6% of the genetic risk for SSNS.36 A common finding from genome-wide association studies of glom­ erular diseases is the significant association with polymorphisms from the major histocompatibility complex. It is not clear whether the HLA loci are causal, given the commonality among glomerular studies, or it reflects the allele frequency by specific ethnicity studied.36 Systemic circulating factors Genetics and SRNS A circulating glomerular permeability factor has been hypothesised to cause NS, however, defining a single putative factor remains elusive. The majority of studies favour a circulating factor in SRNS or FSGS (figure 2). A few studies demonstrate proof of a blood-derived glomerular permeability factor. For example, serum from patients with FSGS in pre-clinical and ex-vivo studies induced proteinuria or increased permeability of glomeruli to albumin.29 There was also successful treatment of recurrent FSGS after kidney transplantation with immuno­adsorption. Finally, maternal transmission of FSGS confirmed a circulating factor.30 There are num­ erous factors proposed including heparanase, haemo­ pexin, angiopoietin-like 4 (ANGPTL4), cardiotrophin-like cytokine-1 and, more recently, soluble urokinase plasmino­ gen activator receptor (suPAR).31–33 These factors might impact glomerular permeability, possibly through effects on the endothelial cell or podocyte. suPAR affects the podocyte actin cytoskeleton via an interaction with αvβ3-integrin receptor on the surface of podocytes. Increased suPAR con­ centrations were originally described in FSGS;34 however, suPAR as a circulating factor has been refuted since concentrations vary by kidney function, there is an absence of specificity for FSGS, and findings are not easily reproducible.35 Two distinct forms of ANGPTL4, a glycoprotein and acute phase reactant expressed in adipose tissue, the heart, and skeletal muscle, are elevated in NS.32 A hypo­ sialylated form secreted from podocytes can lead to Identifying genetic causes for children with SRNS early in their course could allow discontinuation of immuno­ suppressive agents, aid in transplant management, and provide information for prenatal counselling. Con­ firmation of a genetic defect generally implies a reduced risk of recurrence afer transplantation, as it is likely a kidney-specific disease. De novo auto-immune-mediated NS can develop in the transplanted kidney owing to neoantigens,37 especially among children with NPHS1 (nephrin) mutations.38 Depending on the age of onset of SRNS, the likelihood of defining a monogenic cause decreases as children age. Mutations in key podocyte genes such as NPHS1, NPHS2, LAMB2, or WT1 explain 69–85% of cases of NS presenting in the first 3 months of life and 50–66% of NS cases presenting between 4 and 12 months.39,40 After 1 year of age, the chance of identifying a genetic cause for SRNS decreases substantially to 25% between the ages of 1 and 6 years, 18% between 7 and 12 years, and as low as 11% in those aged 13–18 years.39 In an international registry of over 1340 children with SRNS over 1 year of age, approximately 14% had a genetic mutation.41 Over 30 genes are reported to be associated with SRNS or FSGS, and the list is expected to expand (appendix). Mutations in the mitochondrial genes involved in biosynthesis of coenzyme Q10 leading to deficiency, occur in 1% of cases of familial SRNS.39 Coenzyme Q10 is an antioxidant and an essential component of the electron chain and, most importantly, supplementation can be effective for mitochondrial podocytopathies—a potentially www.thelancet.com Vol 392 July 7, 2018 See Online for appendix 65 Seminar curative therapy. High-throughput next generation DNA sequen­cing from patients with sporadic FSGS, targeting all known podocyte genes, might identify other FSGS susceptibility genes.42 Complications of nephrotic syndrome Infection Infection is the leading cause of morbidity and, historically, mortality in children with NS.43 NS is associated with low concentrations of immunoglobulin G (IgG) from urinary loss and altered production, which contributes to infection risk. Loss of complement can also predispose to infection risk. Spontaneous bacterial peritonitis, especially by Streptococcus pneumoniae, remains a serious complication of NS, and a low serum albumin (<15 g/L or 1∙5 g/dL) is associated with increased risk of peritonitis.44 Routine antibiotic prophylaxis for prevention of spontaneous bacterial peritonitis in children with active NS is generally not recommended owing to scarcity of evidence, although some centres could opt for prophylaxis in children with a history of peritonitis.45 Pneumococcal vaccination can successfully be given to children with NS, even when on steroid therapy, and is recommended by the Children’s Nephrotic Syndrome Consensus Con­ ference.46,47 Children with NS are also at risk of developing pneumonia from Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, and cellulitis, caused by Staphylococci, group A Streptococci, and H influenzae species.45 Varicella-zoster infection poses a substantial risk to children with NS. The vaccine is a weakened form of the virus, which is typically best avoided in immuno­ compromised children. It appears to be safe, however, for children who are in remission or who are on low-dose alternate-day steroids, an optimised, two-dose vaccination schedule has been developed.48 Prophylactic varicella-zoster immune globulin (VZIG) is rec­ ommended for exposure to chickenpox. VZIG should be given as soon as possible but can be given up to 10 days after exposure. Therapeutic intravenous aciclovir could also be effective in NS, however, data are scarce and extrapolated from solid organ transplant­ ation reports.49 Venous thromboembolism NS is a well recognised hypercoagulable state in which children are at risk of venous thrombo­ embolism (VTE) including cerebral sinus venous throm­ bosis, pulmonary embolism, and renal vein throm­bosis.50 VTE complicates an estimated 3% of cases of NS during childhood.50,51 The pathophysiology of VTE in NS is multi­ factorial resulting from abnormalities in platelet aggre­ gation, increased synthesis of prothrombotic factors (factors V and VIII), urinary loss of anticoagulant proteins (antithrombin III, protein C and S), altered fibrinolysis, and intra­vascular fluid depletion.52 Treatment 66 is with low-molecular-weight heparin. There is in­ sufficient evidence to warrant universal thrombosis prophylaxis in childhood NS.50 Acute kidney injury (AKI) AKI, an underappreciated complication, is now rec­ognised as the third most important complication in children treated in hospital with NS.53 Recently, a multicentre study from the USA reported that 58∙6% of 336 children admitted to hospital for NS had evidence of AKI with identified risk factors such as concomitant infections, use of nephrotoxic medications and SRNS.53 The use of diuretics in a child with haemoconcentration and intra­ vascular volume depletion might predispose to AKI. Renal vein thrombosis, acute tubular necrosis in the setting of hypovolaemia and sepsis, and interstitial nephritis in­ duced by non-steroidal anti-inflammatory drugs or antibiotics are also recognised contributors to AKI. Dyslipidaemia NS is associated with substantial abnormalities in lipid metabolism, leading to hypercholesterolaemia, hyper­ triglyceridaemia, and various other lipoprotein abnor­ malities. Lipid abnormalities are primarily related to urinary losses of key transport proteins including albumin, which carries free cholesterol, and also a com­ pen­ satory increase in proteins involved in triglyceride metabolism.54 It is unknown whether altered lipid metabolism confers long-term cardiovascular risk from atherosclerosis in children with NS. The use of lipidlowering agents for the dyslipidaemia in NS is not advised, unless there is substantial persistent proteinuria with extremely high levels of hypertriglyceridaemia. The evidence of benefit is not clear and side-effects such as liver dysfunction, risk of rhabdomyolysis, and delayed growth and development although rare are not in­ substantial.55 If statins are initiated, it is only recommended for children over the age of 10 years with monitoring of liver function and creatinine kinase prior to initiating therapy and after 4 weeks.56 Management Oedema in nephrotic syndrome Based on the pathogenesis of oedema in NS, one of the primary strategies in the management of oedema is salt and fluid restriction with addition of a loop diuretic for severe or symptomatic oedema.57 The addition of an albumin infusion, typically in combination with a loop diuretic, is sometimes employed to induce diuresis and natriuresis, especially if signs of intravascular underfilling or severe oedema are present. Response to diuretics alone might be blunted, especially in an underfilled state, where there is activation of neurohormonal systems in an attempt to maintain intravascular volume. Furthermore, furo­ semide is highly protein bound and, in a hypoalbuminaemic state, the volume of distribution substantially increases with less drug available to reach the proximal tubule for www.thelancet.com Vol 392 July 7, 2018 Seminar secretion into the urinary space. The combined use of albumin and furosemide remains controversial. Many studies involved small numbers of patients, were largely uncontrolled, and, in most, only a small benefit of diuresis, natriuresis, and weight loss were observed with the combination of furosemide and albumin over furosemide alone. Approximately 25% of children with NS might actually be hypovolaemic at presentation, as assessed by clinical, laboratory, and echocardiographic parameters.58 Diuretics alone might aggravate hypovolaemia and intravascular depletion, thus, the use of albumin could be warranted. Care should be taken with the administration of albumin in children who are not exhibiting signs of hypovolaemia, as the resultant expansion in intravascular fluid could precipitate pulmonary oedema.57 Management of nephrotic syndrome Standardising management of NS has been hampered by the paucity of high-quality trial evidence supporting international guidelines. This lack of evidence has resulted in substantial variation in care based on physicians’ preference, drug availability by country, and interpretation of the sparse trial data. Substantial physician variability in choice of steroid-sparing agents for children with NS is described in Europe, Canada, and the USA.59,60 In the following section, we outline the principal treatments for NS (figure 3). Certain agents, such as mizoribine, or chlorambucil with restricted availability are not discussed in detail.1 Baseline and additional investigations, as well as considerations for genetic testing and renal biopsy are presented in panel 3. Initial therapy Standard therapy is at least 4–6 weeks of steroids each day (prednisone or prednisolone), followed by a mini­ mum of 6 weeks of alternate day therapy based on evidence from clinical trials.1 Many published protocols by guideline, centre, or country highlight the variability in the prednisone taper (table). Until recently, a Cochrane review strongly suggested that 6 months of therapy was superior to 2–3 months, owing to a substantial reduction in relapses after initial presentation of NS. The meta-analysis of randomised controlled trials (RCTs) involved over 700 children.64 Since 2013, three RCTs demonstrated that extending treatment to 6 months compared with 2–3 months did not reduce the risk of relapse, the development of frequent relapses, or steroid dependence and the eventual need for an additional immunosuppressive agent.65–67 Thus, treatment with steroids for 6 months is no longer recommended.68 Chronic steroid use is associated with many side-effects including obesity, cushingoid features, striae, ocular complications, such as cataracts and glaucoma, metabolic features, musculoskeletal features, such as osteoporosis and avascular necrosis of the head of the femur, and behavioural features. In those that continue to relapse www.thelancet.com Vol 392 July 7, 2018 FRNS or SDNS SSNS Low dose alternate day steroids Infrequent relapses Ongoing relapses or steroid toxicity Cyclophosphamide or levamisole Long-term remission Steroids No response after 8 weeks of steroids SRNS Ongoing relapses or steroid toxicity Biopsy every 2–3 years Tacrolimus or mycophenolate mofetil Ongoing relapses or steroid toxicity Biopsy every 2–3 years Rituximab ± other immunosuppressants Tacrolimus Genetic cause confirmed No response ACE inhibitor ARB Progression over time to ESRD Figure 3: Principal treatments for nephrotic syndrome at diagnosis and follow-up After initial therapy with steroids, children are classified as having SSNS or SRNS after at least 8 weeks of therapy. Frequently relapsing SSNS can first be treated with low-dose alternate-day steroids prior to consideration of steroid-sparing agents. Typically, the steroid-sparing agents include cyclophosphamide, or levamisole, and if this fails and the child continues to relapse or is steroid dependent, then a calcineurin inhibitor is often the next agent, or mycophenolate mofetil. The scarcity of trial evidence has resulted in substantial variation in choice of steroid-sparing agents based on physicians’ preference, drug availability by country, and cost. SSNS=steroid sensitive nephrotic syndrome. SRNS=steroid resistant nephrotic syndrome. FRNS=frequently relapsing nephrotic syndrome. SDNS=steroid dependent nephrotic syndrome. ACE=angiotensin converting enzyme inhibitor. ARB=angiotensin receptor blocker. and require steroids into adulthood, osteo­ porosis and being overweight cause substantial morbidity.9 Treatment of frequently relapsing nephrotic syndrome (FRNS) or steroid-dependent nephrotic syndrome (SDNS) For relapses of SSNS, the efficacy of steroids is not in doubt; however, tapering schedules remains controversial, as data are scarce.1,64 KDIGO recommends a tailored approach to steroid therapy depending on whether children relapse either infrequently or frequently (table).1 Triggers of relapse Additional factors triggering relapses include allergies and infection. Treatment for allergies with dietary restrictions, skin desensitisation, mast cell stabilisers such as disodium cromoglycate demonstrate little benefit in preventing relapsing disease.69 On the contrary, several trials suggest that relapses might be reduced if prednisolone is administered daily for 5–7 days at the onset of upper respiratory tract infection in FRNS or SDNS. Although studies have a high risk of bias owing to small sample sizes and variability in study design, treatment is still recommended by the KDIGO guide­ lines.1,70–72 A large, placebo-controlled, multicentre trial of 300 children in the UK (PREDNOS 2) will clarify the effectiveness and safety of this approach when completed.73 67 Seminar Year of publication International Arbeitsgemeinschaft Study of Kidney für Pädiatrische Disease in Children Nephrologie (APN)2 (ISKDC)61 Haute Autorité de Santé (France)62 Italian Society for Pediatric Nephrology (SINePe)63 KDIGO Glomerulonephritis Guidelines1 Hospital for Sick Children (Toronto, Canada)11 1970 2008 2017 2012 2016 60 mg/m² per day × 6 weeks (maximum 60 mg in single or 2 divided doses) 60 mg/m² per day or 2 mg/kg per day × 4–6 weeks (maximum 60 mg) 60 mg/m² per day × 6 weeks (maximum 60 mg in single morning dose) 60 mg/m² per alternate day × 8 weeks (maximum 60 mg) followed by a 15 mg/m² per alternate day × 15 days and continue to wean. In addition, 3 methylprednisolone pulses if proteinuria persists after 1 month of daily prednisone therapy 40 mg/m² per alternate day × 6 weeks (single dose; maximum 40 mg) without tapering 40 mg/m² per alternate day or 1∙5 mg/kg/alternate day (maximum 40 mg ) × 6–8 weeks (at least 12 weeks) and continued for 2–5 months with tapering 40 mg/m² per alternate day × 6 weeks (maximum 60 mg), 30 mg/m² per alternate day × 8 days (maximum 30 mg), 20 mg/m² per alternate day × 8 days (maximum 20 mg), 10 mg/m² per alternate day × 12 days (maximum 10 mg) 1988 Initial presentation 60 mg/m² per day × 4 weeks 60 mg/m² per day × 6 weeks (maximUm (maximum dose 60 mg) dose 80 mg) Initial dose and duration 60 mg/m² per day × 4 weeks Subsequent dose and tapering 40 mg/m² per 4 weeks of alternate day × 6 weeks 40 mg/m² per alternate day but (maximum dose 60 mg) given on 3 consecutive days out of a week Relapses Starting dose and duration ·· ·· 60 mg/m² per day until urine protein is negative for 6 days 60 mg/m² (max 60 mg in a single or 2 divided doses) until urine protein is negative for 5 days 60 mg/m² per day or 2∙0 mg/kg per day (maximum of 60 mg/day) until urine is negative for 3 days 60 mg/m² per day until urinary protein is trace or negative for 5 consecutive days Follow-up dose and duration ·· ·· 60 mg/m² per alternate day × 4 weeks, 45 mg/m² per alternate day × 4 weeks, 30 mg/m² per alternate day × 4 weeks, 15 mg/m² per alternate day × 4 weeks 40 mg/m² per alternate day (max 40 mg) × 4 weeks 40 mg/m² or 1∙5 mg/kg/ alternate day (maximum 40 mg) × 4 weeks (minimum) 60 mg/m² per alternate day × 8 days (maximum 60 mg/day), 50 mg/m² per alternate day × 8 days (maximum 50 mg/day), 40 mg/m² per alternate day × 8 days (maximum 40 mg/day), 30 mg/m² per alternate day × 8 days (maximum 30 mg/day), 20 mg/m2/alternate day × 8 days (maximum 20 mg/day), 10 mg/m2 per alternate day × 8 days (maximum 10 mg/day) ·· ·· Frequent relapses ·· ·· 60 mg/m2 per day or 2∙0 mg/kg per day (maximum of 60 mg/day) until urine is negative for 3 days followed by alternate-day prednisone for at least 3 months; use the lowest dose to maintain remission without major adverse effects and daily if alternate day is ineffective ·· Table: Published protocols for steroid treatment (prednisone or prednisolone) for initial presentation of idiopathic nephrotic syndrome Low-dose, alternate-day steroid In general, the first step in managing a child with frequent relapses is to maintain them on low-dose, alternate-day steroid, typically at the lowest dose possible, or just above the steroid dose associated with the latest relapse. Children could be treated with steroid-sparing agents when low-dose alternative-day steroid therapy fails or when severe adverse effects of steroids develop (figure 3). The decision to choose steroid-sparing treat­ ments should be based on drug efficacy, side-effects and the patient’s condition. Also, regional availability of 68 medications and physician preferences greatly influence choice of steroid-sparing medication (panel 4).59,60 We provide the list of agents below based on the strength of evidence (figure 3). Steroid-sparing agents Levamisole, an antihelminthic agent, has immuno­ modulatory properties and a favourable side-effect profile. Levamisole reduced the risk of relapse compared with placebo or no treatment.74,75 There was, however, con­ siderable heterogeneity in study design described in a www.thelancet.com Vol 392 July 7, 2018 Seminar meta-analysis.76 An international, multicentre, doubleblind, placebo-controlled, RCT of levamisole in SSNS found that 6% of patients on placebo versus 26% on levamisole were in remission at 1 year.103 Cyclophosphamide, the most commonly used steroidsparing agent, is effective in multiple RCTs for the treatment of FRNS and SDNS.78–81 A Cochrane review reported that cyclophosphamide substantially reduced the relapse risk at 6–12 months by 56% when compared to prednisolone alone (relative risk [RR] 0∙44, 95% CI 0∙26–0∙73).76 A meta-analysis reported that studies for non-steroid-dependent FRNS resulted in remission in 72% of patients after 2 years and 36% after 5 years, whereas the proportions for SDNS were 40% and 24%, respectively.43 Studies to date used various definitions of response, which leads to hetereogeneity in re­ ported outcomes. Important side-effects of cyclophosphamide include gonadal dysfunction (azoospermia in boys), myelo­ suppression (leucopenia), infection, alopecia, haemor­ rhagic cystitis, and hepatic dysfunction. Meta-analyses report that risk of azoospermia is higher in boys of pubertal age (Tanner stage 2 or greater) or post-pubertal age, especially when the cumulative dose of cyclo­ phosphamide ranges from 100 to 300 mg/kg, but higher doses could be safe in pre-pubertal boys.43,104 In NS, substantially lower doses of cyclophosphamide are prescribed typically, 2–2∙5 mg/kg on average for 8–12 weeks, with a maximum daily dose of 100 mg and only as a single course.1 The risk of female infertility is documented to be lower than in boys, and a cumulative dose up to 200 mg/kg is reported safe105 as infertility occurred at a dose of 300 mg/kg or higher.104 Approxi­ mately 32% of NS patients developed leucopenia, during cyclophosphamide therapy.43 Therefore, if the leucocyte count drops below 4500 cells per μL, a lower dose could be used or suspended if the leucocyte count falls below 3000 cells per μL.43 Long-term studies with decades of follow-up are needed to understand long-term risk of cyclophosphamide. Panel 4: Steroid-sparing agents for frequently relapsing nephrotic syndrome (FRNS) or steroid-dependent nephrotic syndrome (SDNS) Levamisole • Reduces risk of relapse compared with placebo or no treatment;74,75 there was, however, considerable heterogeneity in the meta-analysis76 Cyclophosphamide • Effective for FRNS,76,77,79 but less so for steroid-dependent nephrotic99 syndrome;43,79–81,82 however, there is overlap in the classification Ciclosporin • Effective for FRNS or SDNS,83–90 but many patients suffer relapses after discontinuation of ciclosporin therapy (ciclosporin dependence)85,86,89–91 Tacrolimus • Several case series suggest that tacrolimus is effective for FRNS or SDNS,92–96 but there are no randomised controlled trials Mycophenolate mofetil • Less effective than ciclosporin,97,98 but has a favourable side-effect profile Rituximab • Rituximab and lower doses of prednisone and calcineurin inhibitors are non-inferior to standard doses of these agents in maintaining short-term remission in children who show dependence on both drugs, and allow their temporary withdrawal99 • Effective for complicated FRNS or SDNS, but all children had relapses by 19 months after rituximab infusion100 • Non-inferior to steroids in maintaining remission in SDNS101 Mizoribine • Not effective for SDNS, but a subgroup analysis of children aged 10 years and younger demonstrated that the proportion of patients who relapsed was substantially lower in the mizoribine group than the placebo group102 Calcineurin inhibitors Ciclosporin is effective for both FRNS and SDNS.83–87 Blood concentration of ciclosporin should be monitored, usually by trough levels or C2 (at 2 h post-dose) levels, and the dose should be adjusted within target levels (trough levels: 60–100 ng/mL; C2 levels: 300–700 ng/mL).83,84,88 There is, however, no international consensus on target con­ centrations owing to incon­ clusive evidence.1 In Japan, dose is adjusted to maintain trough concentrations within 80–100 ng/mL for the first 6 months, and within 60–80 ng/mL for the next 18 months, and in North America, reported levels range from 50 to 100 ng/ml.85,86,91 Additionally, the length of therapy is not well defined as children frequently relapse after discontinuation of the drug (ciclosporin dependence).85,86,91 Nephrotoxicity is also problematic with www.thelancet.com Vol 392 July 7, 2018 increasing risk after prolonged use of the drug for 2 years or more.106,107 Tacrolimus could be considered when ciclosporin cannot be used owing to cosmetic side-effects, including hypertrichosis and gingival hypertrophy. Both medi­ cations are now generic, thus cost should not be an issue. Potential onset of diabetes and nephrotoxicity are important side-effects. No definitive dosing for tacro­ limus is established, but it typically starts at 0∙1 mg/kg per day (range 0∙05–0∙2).97,98,108 Optimal trough con­ centrations are not defined but ranges from 5 to 8 ng/mL have been reported, and persistent levels greater than 8 ng/mL were associated with increased risk of nephrotoxicity in a single study.93,94 Hypertension could also be worsened with calcineurin inhibitors and 69 Seminar could potentiate the development of posterior reversible encephalopathy syndrome.108 Mycophenolate mofetil (MMF) Mycophenolate mofetil (MMF) is used as a steroidsparing agent for FRNS or SDNS owing to the favourable side-effect profile and absence of nephrotoxicity. Physician preference for MMF stems from the concerns about the toxicity of cyclophosphamide and calcineurin inhibitors; however, trial evidence is scarce. A randomised, multi­centre, open-label, crossover study comparing the efficacy and safety of a 1-year treatment with MMF or ciclosporin in 60 pediatric patients with FRNS, reported more relapses per patient per year with MMF than with ciclosporin.96 Similar results were reported in another multicentre RCT comparing the efficacy of MMF to that of ciclosporin in 24 children with FRNS and biopsyproven MCD.98 A French study that employed Bayesian techniques and probability in 23 children, found that MMF could reduce the number of relapses and steroid doses, suggesting use of MMF prior to cyclophosphamide or ciclosporin.109 Recent studies demonstrate efficacy at higher doses, which are not routinely monitored and typically used in kidney transplant­ation. Maintaining an area under the curve for myco­phenolic acid, assessed by pharmacokinetic studies higher than 45 mg × h per L, might be associated with less frequent relapses.110 Rituximab Rituximab, a chimeric anti-CD20 monoclonal antibody, is effective and allows for discontinuation or reduction of steroids and other steroid-sparing agents in NS. An initial open-label RCT concluded that rituximab and lower doses of prednisone and calcineurin inhibitors are non-inferior to standard therapy in maintaining shortterm re­mission.99 Findings were confirmed by a multi­ centre, double-blind, randomised, placebo-controlled trial that assessed efficacy and safety of rituximab versus placebo in 48 children with complicated FRNS or SDNS.100 Those on rituximab received 375 mg/m² body surface area (maximum 500 mg) once weekly for 4 weeks, and the placebo group received placebo at similar frequency. Prednisolone was gradually tapered after remission was achieved. Tapering of ciclosporin was started on day 85, and discontinued by day 169. The 50% relapse-free period (267 vs 101 days; hazard ratio [HR] 0∙267, 95% CI 0∙135–0∙528, p<0∙0001), and the daily steroid dose was substantially lower in those on rituximab than placebo (9∙12 [SD 5∙88] vs 20∙85 [SD 9∙28] mg/m² per day, p<0∙0001) up to 1 year. In follow-up, all children relapsed by 19 months, suggesting that the benefit of rituximab was not permanent. Re­ cently, rituximab was also shown to be non-inferior to steroids in maintaining remission in patients with SDNS never exposed to a calcineurin inhibitor and who had not received either MMF or cyclophosphamide in the preceding 6 months.101 70 Rituximab is generally safe and well tolerated in most children; however, potentially serious adverse events include persistent low B cell and failure to repopulate, depletion of memory B cells , and a risk of hypo­ gammaglobulinaemia, occasionally requiring infusions of immunoglobulin. Rarer adverse events are also report­ ed, including fatal hepatitis induced by reactivation of hepatitis B virus, progressive multifocal leuko­ encephalopathy, pulmonary fibrosis, fulminant myo­ carditis,111 pneumocystis pneumonia,112 immune-mediated ulcerative colitis, and agranulocytosis. Recently, hyper­ sensitivity reactions were reported with autoantibodies to rituximab during the second course of rituximab.113 Despite the benefit of rituximab, residual issues require further study such as the total number of infusions, whether to redose every 6 months or base dose on repopulation of CD20 B cells. Importantly, we need to understand the long-term consequences of rituximab therapy in children as treatment is considered earlier in disease course. Treatment for steroid-resistant nephrotic syndrome Identification of a podocyte gene defect is fundamental to determining treatment response to steroids and calci­ neurin inhibitors as demonstrated in recent studies.114,115 SRNS with no proven genetic mutation is expected to respond with complete remission in up to 60% of cases and with partial remission in up to 19%.114,115 Furthermore, those with no genetic mutation have a substantial advantage in terms of kidney survival over 10 years, with ESRD occurring in 71% of those with a genetic disease versus 29% in those without.114 Calcineurin inhibitors such as ciclosporin and tacro­ limus are recommended as initial therapy for children with SRNS,1,116 with cumulative complete and partial remission in ciclosporin treatment substantially better than placebo.117–119 Also, tacrolimus is similar to ciclosporin in combination with low-dose steroids in inducing remission in patients with SRNS.120 Optimal duration of calcineurin inhibitor therapy is still unknown, although KDIGO guidelines recommended a minimum of 12 months,1 and in clinical practice, can be con­ tinued up to 24 months. Combination therapy in­ volving steroid pulse therapy and ciclosporin could be considered effective in inducing remission of SRNS in extreme cases.121,122 Two RCTs demonstrated a substantial reduction in proteinuria with enalapril123 and fosinopril.105 KDIGO therefore recommended angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers for children with SRNS.1 Cyclophosphamide has no benefit for SRNS.78,124 MMF use in the treatment of SRNS has been described and the proportion of patients in remission is low.125–127 A recent randomised trial of 138 children evaluated dexamethasone plus MMF as a therapy for patients with steroid-resistant FSGS versus ciclosporin, and found no significant www.thelancet.com Vol 392 July 7, 2018 Seminar difference in achieving partial or complete remission at 1 year; however, the trial was substantially under­ powered.128 Several case series suggested that rituximab is effective with refractory (failing to respond to calcineurin inhibitors) SRNS; however, an open-label, randomised trial of rituximab failed to demonstrate an improvement in 31 children with SRNS, compared to either 16 children who received calcineurin inhibitors, prednisolone, and two infusions of rituximab, or 15 children who received calcineurin inhibitors and prednisolone alone.129 Controversies, uncertainties, and outstanding research questions There are many remaining questions about NS and these can be grouped into understanding: (1) who develops NS and what is the cause?; (2) factors contributing to interindividual variability in response to medications; and (3) specific triggers leading to relapsing disease. There are several controversies and uncertainties. What is the expected dose of calcineurin inhibitors that will induce and maintain remission? When should we discontinue calcineurin inhibitors in children who maintain remission? What is the role of MMF and rituximab? Well designed RCTs need to be conducted to clarify these uncertainties. Additionally, there is a need for optimal trials to address novel treatments and tapering regimens, and follow-up studies to address potential longterm risk of medications, especially among those who receive biological agents. For complicated FRNS or SDNS, further modification of rituximab treat­ ment, including adjunct immunosuppressive therapies and repeated courses of rituximab, might be necessary to extend the relapse-free period. Finally, a comparison of the efficacy, safety, and cost-effectiveness of various rituximab dosing regimens and B-cell-driven regimens are needed. There is also a need to develop novel therapies to address refractory disease and FSGS. Several therapies are in the research phase, including sirolimus, apheresis, adalimumab, fresolimumab, rosiglitazone, galactose at high dose, and ofatumumab (appendix). Finally, a determination of the social and patient-centred factors that affect outcomes will aid in counselling children and families about long-term prognosis. Conclusion Although, there is substantial morbidity due to chronic use of steroids and other steroid-sparing agents, less than 5% of children with SSNS progress to ESRD. Steroid resistance, however, is an important determinant of future risk for ESRD.8 Historically, more than 90% of children with NS enter long-term remission after puberty,6,7 however, the precise number is not known, especially in frequent relapsers or those on steroid-sparing agents with the potential of active disease in adulthood.8–10 Contributors DGN and RP conducted the literature search with initial manuscript preparation. All authors contributed equally to data interpretation, www.thelancet.com Vol 392 July 7, 2018 writing of the manuscript, and construction of the figures, panels, and tables. All authors reviewed and approved the final version. Declaration of interests DGN declares no competing interests. KI has received grants from Novartis Pharma, Japan Blood Product Organization, AbbVie, JCR Pharmaceuticals, Daiichi Sankyo, Teijin Pharma, CSL Behring, Novo Nordisk Pharma, Air Water Medical, Astellas Pharma, Takeda Pharmaceutical, Taisho Toyama Pharmaceutical, Eisai, and Biofermin Pharmaceutical, and lecture fees or consulting fees, or both from Zenyaku Kogyo, Novartis Pharma, Chugai Pharmaceutical, Astellas Pharma, Springer Japan, Meiji Seika Pharma, Asahi kasei Pharma Corporation, Medical Review, Nippon Boehringer Ingelheim, Baxter Limited, Ono Pharmaceutical, Sanwa Kagaku Kenkyusho, Sanofi, Alexion Pharma, and Kyowa Hakko Kirin. RP has received grants from Astellas Pharma. References 1 KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2012; 2: 139–274. 2 Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Disease in Children. Kidney Int 1978; 13: 159–65. 3 Arbeitsgemeinschaft fur Padiatrische Nephrologie. Short versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Lancet 1988; 331: 380–83. 4 Koskimies O, Vilska J, Rapola J, Hallman N. Long-term outcome of primary nephrotic syndrome. Arch Dis Child 1982; 57: 544–48. 5 Tarshish P, Tobin JN, Bernstein J, Edelmann CM Jr. Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 1997; 8: 769–76. 6 Trompeter RS, Lloyd BW, Hicks J, White RH, Cameron JS. Long-term outcome for children with minimal-change nephrotic syndrome. Lancet 1985; 325: 368–70. 7 Ruth EM, Kemper MJ, Leumann EP, Laube GF, Neuhaus TJ. Children with steroid-sensitive nephrotic syndrome come of age: long-term outcome. J Pediatr 2005; 147: 202–07. 8 Lewis MA, Baildom EM, Davis N, Houston IB, Postlethwaite RJ. Nephrotic syndrome: from toddlers to twenties. Lancet 1989; 33: 255–59. 9 Fakhouri F, Bocquet N, Taupin P, et al. Steroid-sensitive nephrotic syndrome: from childhood to adulthood. Am J Kidney Dis 2003; 41: 550–57. 10 Skrzypczyk P, Panczyk-Tomaszewska M, Roszkowska-Blaim M, et al. Long-term outcomes in idiopathic nephrotic syndrome: from childhood to adulthood. Clin Nephrol 2014; 81: 166–73. 11 Banh TH, Hussain-Shamsy N, Patel V, et al. Ethnic differences in incidence and outcomes of childhood nephrotic syndrome. Clin J Am Soc Nephrol 2016; 11: 1760–68. 12 Mendonca AC, Oliveira EA, Froes BP, et al. A predictive model of progressive chronic kidney disease in idiopathic nephrotic syndrome. Pediatr Nephrol 2015; 30: 2011–20. 13 Trautmann A, Schnaidt S, Lipska-Zietkiewicz BS, et al. Long-term outcome of steroid-resistant nephrotic syndrome in children. J Am Soc Nephrol 2017; 28: 3055–65. 14 Gipson DS, Chin H, Presler TP, et al. Differential risk of remission and ESRD in childhood FSGS. Pediatr Nephrol 2006; 21: 344–49. 15 Joshi S, Andersen R, Jespersen B, Rittig S. Genetics of steroid-resistant nephrotic syndrome: a review of mutation spectrum and suggested approach for genetic testing. Acta Paediatr 2013; 102: 844–56. 16 Hama T, Nakanishi K, Shima Y, et al. Renal biopsy criterion in idiopathic nephrotic syndrome with microscopic hematuria at onset. Pediatr Nephrol 2015; 30: 445–50. 17 Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal Change Disease. Clin J Am Soc Nephrol 2017; 12: 332–45. 18 D’Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med 2011; 365: 2398–411. 19 Chanchlani R, Parekh RS. Ethnic differences in childhood nephrotic syndrome. Front Pediatr 2016; 4: 39. 20 Boyer O, Moulder JK, Somers MJ. Focal and segmental glomerulosclerosis in children: a longitudinal assessment. Pediatr Nephrol 2007; 22: 1159–66. 71 Seminar 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 72 Akchurin O, Reidy KJ. Genetic causes of proteinuria and nephrotic syndrome: impact on podocyte pathobiology. Pediatr Nephrol 2015; 30: 221–33. Buscher AK, Weber S. Educational paper: the podocytopathies. Eur J Pediatr 2012; 171: 1151–60. Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet 1974; 304: 556–60. Lin CY, Hsu HC. Histopathological and immunological studies in spontaneous remission of nephrotic syndrome after intercurrent measles infection. Nephron 1986; 42: 110–15. Audard V, Larousserie F, Grimbert P, et al. Minimal change nephrotic syndrome and classical Hodgkin’s lymphoma: report of 21 cases and review of the literature. Kidney Int 2006; 69: 2251–60. Reiser J, von Gersdorff G, Loos M, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clinical Invest 2004; 113: 1390–97. Yu CC, Fornoni A, Weins A, et al. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med 2013; 369: 2416–23. Benigni A, Gagliardini E, Remuzzi G. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med 2014; 370: 1261–63. Savin VJ, Sharma R, Sharma M, et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med 1996; 334: 878–83. Kemper MJ, Wolf G, Muller-Wiefel DE. Transmission of glomerular permeability factor from a mother to her child. N Engl J Med 2001; 344: 386–67. Brenchley PE. Vascular permeability factors in steroid-sensitive nephrotic syndrome and focal segmental glomerulosclerosis. Nephrol Dial Transplant 2003; 18 (suppl 6): vi21–25. Clement LC, Mace C, Avila-Casado C, Joles JA, Kersten S, Chugh SS. Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat Med 2014; 20: 37–46. McCarthy ET, Sharma M, Savin VJ. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2010; 5: 2115–21. Wei C, El Hindi S, Li J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 2011; 17: 952–60. Hayek SS, Quyyumi AA, Reiser J. Soluble urokinase receptor and chronic kidney disease. N Engl J Med 2016; 374: 891. Gbadegesin RA, Adeyemo A, Webb NJ, et al. HLA-DQA1 and PLCG2 are candidate risk loci for childhood-onset steroid-sensitive nephrotic syndrome. J Am Soc Nephrol 2015; 26: 1701–10. Patrakka J, Ruotsalainen V, Reponen P, et al. Recurrence of nephrotic syndrome in kidney grafts of patients with congenital nephrotic syndrome of the Finnish type: role of nephrin. Transplantation 2002; 73: 394–403. Kuusniemi AM, Qvist E, Sun Y, et al. Plasma exchange and retransplantation in recurrent nephrosis of patients with congenital nephrotic syndrome of the Finnish type (NPHS1). Transplantation 2007; 83: 1316–23. Sadowski CE, Lovric S, Ashraf S, et al. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol 2015; 26: 1279–89. Hinkes BG, Mucha B, Vlangos CN, et al. Nephrotic syndrome in the first year of life: two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2). Pediatrics 2007; 119: e907–19. Trautmann A, Bodria M, Ozaltin F, et al. Spectrum of steroid-resistant and congenital nephrotic syndrome in children: the PodoNet registry cohort. Clin J Am Soc Nephrol 2015; 10: 592–600. Yu H, Artomov M, Brahler S, et al. A role for genetic susceptibility in sporadic focal segmental glomerulosclerosis. J Clin Invest 2016; 126: 1067–78. Latta K, von Schnakenburg C, Ehrich JH. A meta-analysis of cytotoxic treatment for frequently relapsing nephrotic syndrome in children. Pediatr Nephrol 2001; 16: 271–82. Hingorani SR, Weiss NS, Watkins SL. Predictors of peritonitis in children with nephrotic syndrome. Pediatr Nephrol 2002; 17: 678–82. McCaffrey J, Lennon R, Webb NJ. The non-immunosuppressive management of childhood nephrotic syndrome. Pediatr Nephrol 2016; 31: 1383–402. 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 Ulinski T, Leroy S, Dubrel M, Danon S, Bensman A. High serological response to pneumococcal vaccine in nephrotic children at disease onset on high-dose prednisone. Pediatr Nephrol 2008; 23: 1107–13. Gipson DS, Massengill SF, Yao L, et al. Management of childhood onset nephrotic syndrome. Pediatrics 2009; 124: 747–57. Furth SL, Arbus GS, Hogg R, et al. Varicella vaccination in children with nephrotic syndrome: a report of the Southwest Pediatric Nephrology Study Group. J Pediatr 2003; 142: 145–48. Prelog M, Schonlaub J, Zimmerhackl LB. Aciclovir and varicella-zoster-immunoglobulin in solid-organ transplant recipients. Pediatr Nephrol 2011; 26: 663–73. Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol 2012; 7: 513–20. Kerlin BA, Haworth K, Smoyer WE. Venous thromboembolism in pediatric nephrotic syndrome. Pediatr Nephrol 2014; 29: 989–97. Kerlin BA, Blatt NB, Fuh B, et al. Epidemiology and risk factors for thromboembolic complications of childhood nephrotic syndrome: a Midwest Pediatric Nephrology Consortium (MWPNC) study. J Pediatr 2009; 155: 105–10. Rheault MN, Zhang L, Selewski DT, et al. AKI in children hospitalized with nephrotic syndrome. Clin J Am Soc Nephrol 2015; 10: 2110–18. Vaziri ND. HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat Rev Nephrol 2016; 12: 37–47. Kong X, Yuan H, Fan J, Li Z, Wu T, Jiang L. Lipid-lowering agents for nephrotic syndrome. Cochrane Database Syst Rev 2013; 12: CD005425. National Heart, Lung and, Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011; 128 (suppl 5): S213–56. Teoh CW, Robinson LA, Noone D. Perspectives on edema in childhood nephrotic syndrome. Am J Physiol Renal Physiol 2015; 309: F575–82. Buyukavci MA, Civilibal M, Elevli M, Selcuk Duru HN. Hypo- and hypervolemic edema in children with steroid sensitive nephrotic syndrome. Turk J Med Sci 2015; 45: 178–83. Samuel S, Morgan CJ, Bitzan M, et al. Substantial practice variation exists in the management of childhood nephrotic syndrome. Pediatr Nephrol 2013; 28: 2289–98. Pasini A, Aceto G, Ammenti A, et al. Best practice guidelines for idiopathic nephrotic syndrome: recommendations versus reality. Pediatr Nephrol 2015; 30: 91–101. Abramowicz M, Barnett HL, Edelmann CM Jr, et al. Controlled trial of azathioprine in children with nephrotic syndrome. A report for the international study of kidney disease in children. Lancet 1970; 295: 959–61. Haute Autorité de Santé. Syndrome néphrotique idiopathique de l’enfant. Protocole national de diagnostic et de soins pour une maladie rare. http://www.afpssu.com/wp-content/ uploads/2013/07/NephrotiqueIdiopthiquePNDS.pdf 2008 (accessed March 5, 2018). Pasini A, Benetti E, Conti G, et al. The Italian Society for Pediatric Nephrology (SINePe) consensus document on the management of nephrotic syndrome in children: Part I - Diagnosis and treatment of the first episode and the first relapse. Ital J Pediatr 2017; 43: 41. Hodson EM, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev 2007; 4: CD001533. Teeninga N, Kist-van Holthe JE, van Rijswijk N, et al. Extending prednisolone treatment does not reduce relapses in childhood nephrotic syndrome. J Am Soc Nephrol 2013; 24: 149–59. Yoshikawa N, Nakanishi K, Sako M, et al. A multicenter randomized trial indicates initial prednisolone treatment for childhood nephrotic syndrome for two months is not inferior to six-month treatment. Kidney Int 2015; 87: 225–32. Sinha A, Saha A, Kumar M, et al. Extending initial prednisolone treatment in a randomized control trial from 3 to 6 months did not significantly influence the course of illness in children with steroid-sensitive nephrotic syndrome. Kidney Int 2015; 87: 217–24. Hodson E. The management of idiopathic nephrotic syndrome in children. Paediatr Drugs 2003; 5: 335–49. www.thelancet.com Vol 392 July 7, 2018 Seminar 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 Abdel-Hafez M, Shimada M, Lee PY, Johnson RJ, Garin EH. Idiopathic nephrotic syndrome and atopy: is there a common link? Am J Kidney Dis 2009; 54: 945–53. Mattoo TK, Mahmoud MA. Increased maintenance corticosteroids during upper respiratory infection decrease the risk of relapse in nephrotic syndrome. Nephron 2000; 85: 343–45. Abeyagunawardena AS, Trompeter RS. Increasing the dose of prednisolone during viral infections reduces the risk of relapse in nephrotic syndrome: a randomised controlled trial. Arch Dis Child 2008; 93: 226–28. Gulati A, Sinha A, Sreenivas V, Math A, Hari P, Bagga A. Daily corticosteroids reduce infection-associated relapses in frequently relapsing nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol 2011; 6: 63–69. Webb NJ, Frew E, Brettell EA, et al. Short course daily prednisolone therapy during an upper respiratory tract infection in children with relapsing steroid-sensitive nephrotic syndrome (PREDNOS 2): protocol for a randomised controlled trial. Trials 2014; 15: 147. Levamisole for corticosteroid-dependent nephrotic syndrome in childhood. British Association for Paediatric Nephrology. Lancet 1991; 337: 1555–57. Dayal U, Dayal AK, Shastry JC, Raghupathy P. Use of levamisole in maintaining remission in steroid-sensitive nephrotic syndrome in children. Nephron 1994; 66: 408–12. Pravitsitthikul N, Willis NS, Hodson EM, Craig JC. Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children. Cochrane Database Syst Rev 2013; 10: CD002290. Barratt TM, Soothill JF. Controlled trial of cyclophosphamide in steroid-sensitive relapsing nephrotic syndrome of childhood. Lancet 1970; 296: 479–82. Prospective, controlled trial of cyclophosphamide therapy in children with nephrotic syndrome. Report of the International study of Kidney Disease in Children. Lancet 1974; 304: 423–27. Vester U, Kranz B, Zimmermann S, Hoyer PF. Cyclophosphamide in steroid-sensitive nephrotic syndrome: outcome and outlook. Pediatr Nephrol 2003; 18: 661–64. Zagury A, de Oliveira AL, de Moraes CA, et al. Long-term follow-up after cyclophosphamide therapy in steroid-dependent nephrotic syndrome. Pediatr Nephrol 2011; 26: 915–20. Kemper MJ, Altrogge H, Ludwig K, Timmermann K, Muller-Wiefel DE. Unfavorable response to cyclophosphamide in steroid-dependent nephrotic syndrome. Pediatr Nephrol 2000; 14: 772–75. Garin EH, Pryor ND, Fennell RS III, Richard GA. Pattern of response to prednisone in idiopathic, minimal lesion nephrotic syndrome as a criterion in selecting patients for cyclophosphamide therapy. J Pediatr 1978; 92: 304–48. Ishikura K, Ikeda M, Hattori S, et al. Effective and safe treatment with cyclosporine in nephrotic children: a prospective, randomized multicenter trial. Kidney Int 2008; 73: 1167–73. Ishikura K, Yoshikawa N, Hattori S, et al. Treatment with microemulsified cyclosporine in children with frequently relapsing nephrotic syndrome. Nephrol Dial Transplant 2010; 25: 3956–62. El-Husseini A, El-Basuony F, Mahmoud I, et al. Long-term effects of cyclosporine in children with idiopathic nephrotic syndrome: a single-centre experience. Nephrol Dial Transplant 2005; 20: 2433–38. Tanaka R, Yoshikawa N, Kitano Y, Ito H, Nakamura H. Long-term ciclosporin treatment in children with steroid-dependent nephrotic syndrome. Pediatr Nephrol 1993; 7: 249–52. Inoue Y, Iijima K, Nakamura H, Yoshikawa N. Two-year cyclosporin treatment in children with steroid-dependent nephrotic syndrome. Pediatr Nephrol 1999; 13: 33–38. Iijima K, Sako M, Oba MS, et al. Cyclosporine C2 monitoring for the treatment of frequently relapsing nephrotic syndrome in children: a multicenter randomized phase II trial. Clin J Am Soc Nephrol 2014; 9: 271–78. Niaudet P, Broyer M, Habib R. Treatment of idiopathic nephrotic syndrome with cyclosporin A in children. Clin Nephrol 1991; 35 (suppl 1): S31–36. Kitano Y, Yoshikawa N, Tanaka R, Nakamura H, Ninomiya M, Ito H. Ciclosporin treatment in children with steroid-dependent nephrotic syndrome. Pediatr Nephrol 1990; 4: 474–77. www.thelancet.com Vol 392 July 7, 2018 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 Ishikura K, Yoshikawa N, Nakazato H, et al. Two-year follow-up of a prospective clinical trial of cyclosporine for frequently relapsing nephrotic syndrome in children. Clin J Am Soc Nephrol 2012; 7: 1576–83. Sinha MD, MacLeod R, Rigby E, Clark AG. Treatment of severe steroid-dependent nephrotic syndrome (SDNS) in children with tacrolimus. Nephrol Dial Transplant 2006; 21: 1848–54. Roberti I, Vyas S. Long-term outcome of children with steroid-resistant nephrotic syndrome treated with tacrolimus. Pediatr Nephrol 2010; 25: 1117–24. Butani L, Ramsamooj R. Experience with tacrolimus in children with steroid-resistant nephrotic syndrome. Pediatr Nephrol 2009; 24: 1517–23. Morgan C, Sis B, Pinsk M, Yiu V. Renal interstitial fibrosis in children treated with FK506 for nephrotic syndrome. Nephrol Dial Transplant 2011; 26: 2860–65. Sinha A, Bagga A, Gulati A, Hari P. Short-term efficacy of rituximab versus tacrolimus in steroid-dependent nephrotic syndrome. Pediatr Nephrol 2012; 27: 235–41. Gellermann J, Weber L, Pape L, et al. Mycophenolate mofetil versus cyclosporin A in children with frequently relapsing nephrotic syndrome. J Am Soc Nephrol 2013; 24: 1689–97. Dorresteijn EM, Kist-van Holthe JE, Levtchenko EN, Nauta J, Hop WC, van der Heijden AJ. Mycophenolate mofetil versus cyclosporine for remission maintenance in nephrotic syndrome. Pediatr Nephrol 2008; 23: 2013–20. Ravani P, Magnasco A, Edefonti A, et al. Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol 2011; 6: 1308–15. Iijima K, Sako M, Nozu K, et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2014; 384: 1273–81. Ravani P, Rossi R, Bonanni A, et al. Rituximab in children with steroid-dependent nephrotic syndrome: a multicenter, open-label, noninferiority, randomized controlled trial. J Am Soc Nephrol 2015; 26: 2259–66. Yoshioka K, Ohashi Y, Sakai T, et al. A multicenter trial of mizoribine compared with placebo in children with frequently relapsing nephrotic syndrome. Kidney Int 2000; 58: 317–24. Gruppen MP, Bouts AH, Jansen-van der Weide MC, et al. A randomized clinical trial indicates that levamisole increases the time to relapse in children with steroid-sensitive idiopathic nephrotic syndrome. Kidney Int 2017; 93: 510–18. Rivkees SA, Crawford JD. The relationship of gonadal activity and chemotherapy-induced gonadal damage. JAMA 1988; 259: 2123–25. Yi Z, Li Z, Wu XC, He QN, Dang XQ, He XJ. Effect of fosinopril in children with steroid-resistant idiopathic nephrotic syndrome. Pediatr Nephrol 2006; 21: 967–72. Iijima K, Hamahira K, Tanaka R, et al. Risk factors for cyclosporine-induced tubulointerstitial lesions in children with minimal change nephrotic syndrome. Kidney Int 2002; 61: 1801–05. Fujinaga S, Kaneko K, Muto T, Ohtomo Y, Murakami H, Yamashiro Y. Independent risk factors for chronic cyclosporine induced nephropathy in children with nephrotic syndrome. Arch Dis Child 2006; 91: 666–70. Ishikura K, Ikeda M, Hamasaki Y, et al. Nephrotic state as a risk factor for developing posterior reversible encephalopathy syndrome in paediatric patients with nephrotic syndrome. Nephrol Dial Transplant 2008; 23: 2531–36. Baudouin V, Alberti C, Lapeyraque AL, et al. Mycophenolate mofetil for steroid-dependent nephrotic syndrome: a phase II Bayesian trial. Pediatr Nephrol 2012; 27: 389–96. Hackl A, Cseprekal O, Gessner M, et al. Mycophenolate mofetil therapy in children with idiopathic nephrotic syndrome: does therapeutic drug monitoring make a difference? Ther Drug Monit 2016; 38: 274–79. Sellier-Leclerc AL, Belli E, Guerin V, Dorfmuller P, Deschenes G. Fulminant viral myocarditis after rituximab therapy in pediatric nephrotic syndrome. Pediatr Nephrol 2013; 28: 1875–79. Sato M, Ito S, Ogura M, et al. Atypical Pneumocystis jiroveci pneumonia with multiple nodular granulomas after rituximab for refractory nephrotic syndrome. Pediatr Nephrol 2013; 28: 145–49. 73 Seminar 113 Ahn YH, Kang HG, Lee JM, Choi HJ, Ha IS, Cheong HI. Development of antirituximab antibodies in children with nephrotic syndrome. Pediatr Nephrol 2014; 29: 1461–64. 114 Buscher AK, Kranz B, Buscher R, et al. Immunosuppression and renal outcome in congenital and pediatric steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol 2010; 5: 2075–84. 115 Buscher AK, Beck BB, Melk A, et al. Rapid response to cyclosporin A and favorable renal outcome in nongenetic versus genetic steroidresistant nephrotic syndrome. Clin J Am Soc Nephrol 2016; 11: 245–53. 116 Hodson EM, Willis NS, Craig JC. Interventions for idiopathic steroid-resistant nephrotic syndrome in children. Cochrane Database Syst Rev 2010; 11: CD003594. 117 Garin EH, Orak JK, Hiott KL, Sutherland SE. Cyclosporine therapy for steroid-resistant nephrotic syndrome. A controlled study. Am J Dis Child 1988; 142: 985–88. 118 Lieberman KV, Tejani A. A randomized double-blind placebo-controlled trial of cyclosporine in steroid-resistant idiopathic focal segmental glomerulosclerosis in children. J Am Soc Nephrol 1996; 7: 56–63. 119 Ponticelli C, Rizzoni G, Edefonti A, et al. A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney Int 1993; 43: 1377–84. 120 Choudhry S, Bagga A, Hari P, Sharma S, Kalaivani M, Dinda A. Efficacy and safety of tacrolimus versus cyclosporine in children with steroid-resistant nephrotic syndrome: a randomized controlled trial. Am J Kidney Dis 2009; 53: 760–69. 121 Hamasaki Y, Yoshikawa N, Hattori S, et al. Cyclosporine and steroid therapy in children with steroid-resistant nephrotic syndrome. Pediatr Nephrol 2009; 24: 2177–85. 74 122 Waldo FB, Benfield MR, Kohaut EC. Therapy of focal and segmental glomerulosclerosis with methylprednisolone, cyclosporine A, and prednisone. Pediatr Nephrol 1998; 12: 397–400. 123 Bagga A, Mudigoudar BD, Hari P, Vasudev V. Enalapril dosage in steroid-resistant nephrotic syndrome. Pediatr Nephrol 2004; 19: 45–50. 124 Tarshish P, Tobin JN, Bernstein J, Edelmann CM, Jr. Cyclophosphamide does not benefit patients with focal segmental glomerulosclerosis. A report of the International Study of Kidney Disease in Children. Pediatr Nephrol 1996; 10: 590–93. 125 de Mello VR, Rodrigues MT, Mastrocinque TH, et al. Mycophenolate mofetil in children with steroid/cyclophosphamide-resistant nephrotic syndrome. Pediatr Nephrol 2010; 25: 453–60. 126 Li Z, Duan C, He J, et al. Mycophenolate mofetil therapy for children with steroid-resistant nephrotic syndrome. Pediatr Nephrol 2010; 25: 883–88. 127 Gargah TT, Lakhoua MR. Mycophenolate mofetil in treatment of childhood steroid-resistant nephrotic syndrome. J Nephrol 2011; 24: 203–07. 128 Gipson DS, Trachtman H, Kaskel FJ, et al. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int 2011; 80: 868–78. 129 Magnasco A, Ravani P, Edefonti A, et al. Rituximab in children with resistant idiopathic nephrotic syndrome. J Am Soc Nephrol 2012; 23: 1117–24. © 2018 Elsevier Ltd. All rights reserved. www.thelancet.com Vol 392 July 7, 2018