- Ninguna Categoria
TABLA ENTALPIA,ENTROPIA,ENERGIA LIBRE
Anuncio
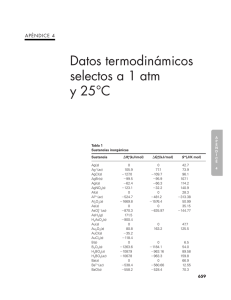
AUXILIAR DE DOCENCIA: Univ. Miguel Angel Gutierrez FISICOQUIMICA MAGNITUDES TERMODINÁMICAS PARA SUSTANCIAS E IONES A 25º C SUSTANCIA e - (g) H + (g) H + (ac) H(g) H2(g) ∆H O ( KJ / mol ) ∆G O ( KJ / mol ) S O ( J / mol − K ) 0 1536.3 0 218.0 0 0 1517.1 0 203.30 0 20.87 108.83 0 114.60 130.6 687.163 -278.46 161 0 -616.9 -408 -351 -270 609.839 -239.66 107.76 0 -575.4 -411.1 -361 -288 -947.7 -1130.8 514.197 -251.2 89.2 0 -568.6 -436.68 -394 -328 495.04 -246 85.81 0 -549.28 -430.58 -389.2 -328 458.5 -248 76.7 0 -554.7 -442.8 -395 -337 649.989 -293.8 128 0 -558.7 -384 -342 -270 574.877 -261.87 77.299 0 -545.1 -384.0 -349 -285 -851.9 -1048.1 481.202 -282.28 60.7 0 -538.9 -408.8 -380 -323 -282.2 55.86 0 132.91 14 138.67 29.10 35.66 59.30 74.1 85.8 147.85 60.2 153.61 51.446 51.21 72.12 86.82 98.5 102 139 154.47 103 160.23 64.672 66.55 82.55 95.94 106.39 124 169.99 69.5 -378.1 -326 427.1 -282.0 49.7 0 -525.4 -414 -383 -333 108.3 118.0 169.72 133 175.5 85.15 88 101.18 121 130 Grupo IA Li + (g) Li + (ac) Li (g) Li (s) LiF (s) LiCI (s) LiBr (s) Li I (s) Na + (g) Na + (ac) Na (g) Na (s) NaF (s) Na Cl (s) Na Br (s) Na I (s) Na HCO 3 (s) Na 2 CO 3 (s) K + (g) K+ (ac) K (g) K(s) KF (s) KCI (s) KBr (s) KI (s) Rb + (g) Rb + (ac) Rb +(g) Rb +(s) Rb F(s) Rb CI(s) Rb Br(s) Rb I(s) Cs + (g) Cs + (ac) Cs +(g) Cs +(s) Cs F(s) Cs CI(s) Cs Br(s) Cs I(s) Grupo IIA Mg 2+ (g) 2351 AUXILIAR DE DOCENCIA: Univ. Miguel Angel Gutierrez FISICOQUIMICA 1 AUXILIAR DE DOCENCIA: Univ. Miguel Angel Gutierrez Mg 2+(ac) Mg + (g) Mg (g) Mg (s) Mg CI2 (s) Mg O (s) Mg 3 N2 (s) Mg CO3 (s) Ca2+ (g) Ca 2+(ac) Ca + (g) Ca (g) Ca (s) Ca F2 (s) Ca CI2 (s) Ca O (s) Ca CO3 (s) Ca SO4 (g) Ca3(PO4) 2 (s) Sr2+ (g) Sr 2+(ac) Sr+ (g) Sr (g) Sr (s) Sr CI2 (s) Sr O (s) Sr CO3 (s) Sr SO4 (s) Ba 2+(g) Ba 2+ (ac) Ba+ (g) Ba (g) Ba (s) BaCI 2 (s) Ba O (s) Ba CO3 (s) Ba SO4(s) FISICOQUIMICA -456.01 -118 115 0 -592.1 -569.0 -401 -1028 148.55 32.69 89.630 26.9 88 65.86 -553.04 -55.2 158.9 0 -1162 -750.2 -603.5 -1128.8 -1320.3 -3899 154.78 41.6 68.87 114 38.2 92.9 107 263 -557.3 -39 110 0 -781.2 -562.4 -1138 -1334 164.54 54.4 117 55.5 97.1 122 -560.7 13 144.8 0 -810.9 -520.4 -1139 -1353 170.28 62.5 126 72.07 112 132 0 -1272 0 -524.7 -1676 0 -1193 0 -481.2 -1582 5.87 53.8 28.3 -313 50.94 715.0 0 1.896 -110.5 -393.5 412.9 -676.26 -691.11 669.6 0 2.866 137.2 -394.4 -386.2 -528.10 -587.06 158.0 5.686 2.439 197.5 213.7 121 -53.1 95.0 -461.96 894.1 150 0 -641.6 -601.2 -461 -1112 1934.1 -542.96 788.6 192.6 0 -1215 -795.0 -635.1 -1206.9 -1432.7 -4138 1784 -545.51 719.6 164 0 -828.4 -592.0 -1218 -1445 1649.9 -538.36 684.6 175.6 0 -806.06 -548.1 -1219 -1465 Grupo IIIA B ((b) – romboédrico) B2O2(s) Al (s) Al3+Cac) Al2O3(s) Grupo IVA C(g) C(grafito) C (diamantes) CO(g) CO2(g) CO2 (ac) CO32-(ac) HCO3 – (ac) AUXILIAR DE DOCENCIA: Univ. Miguel Angel Gutierrez FISICOQUIMICA 2 AUXILIAR DE DOCENCIA: Univ. Miguel Angel Gutierrez H2CO3(ac) CH4(g) C2H2 (g) C2H4 (g) C2H6 (g) C6H6 (l) CH3OH(g) CH3OH (l) HCHO(g) HCOO- (a) HCOOH (l) HCOOH (ac) C2H5 OH (l) CH3 CHO (g) CH3 COOH (l) CN- (ac) HCN(g) HCN(l) HCN(ac) CS2 (g) CS 2 (l) CH3 CI (g) CH2Cl2 (l) CHCl3 (l) CCI4 (g) CCI4 (l) COCI2 (g) Si (s) SiO2 (s) Sn (gris) Sn (blanco) Sn Cl4 (l) Pb2+ (ac) Pb (s) PbO (s, rojo) PbO2(s) PbS(s) PbCl2(s) PbSO4(s) FISICOQUIMICA -698.7 -74.87 227 52.47 -84.667 49.0 -201.2 -238.6 -116 -410 -409 -410 -277.63 -166 -487.0 151 135 105 105 117 87.9 -83.7 -117 -132 -96.0 -139 -220 0 -910.9 3 0 -545.2 1.6 0 -219.0 -276.6 -98.3 -359 -918.39 -623.42 -50.81 209 68.36 -32.89 124.5 -161.9 -166.2 -110 -335 -346 -356 -174.8 -133.7 -392 166 125 121 112 66.9 63.6 -60.2 -63.2 -71.5 -53.7 -68.6 -206 0 -86.5 4.6 0 -474.0 -24.3 0 -189.2 -219.0 -96.7 -314 -811.24 191 186.1 200.85 218.22 229.5 172.8 238 127 219 91.6 129.0 164 161 266 160 118 201.7 112.8 129 237.79 151.0 234 179 203 309.7 214.4 283.74 18.0 41.5 44.8 51.5 259 21 64.785 66.5 76.6 91.3 136 147 473 0 90.29 33.2 9.16 11 -45.9 -80.83 -206.57 -173.23 -206.57 -365.6 333.9 0 69.8 146.2 456 0 86.60 51 97.7 118 -16 26.7 -110.5 -79.914 -110.5 -184.0 292.0 0 48.0 103.8 153.2 191.5 210.65 239.9 304.3 346 193 110 146 155.6 146 151.1 163.1 22.8 164 218.0 Grupo VA N(g) N2(g) NO(g) NO2(g) N2O4(g) N2O5(g) NH3(g) NH3(ac) NO3-(ac) HNO3(l) HNO3(ac) NH4NO3(s) P (g) P(rojo) P4(blanco) P2(g) AUXILIAR DE DOCENCIA: Univ. Miguel Angel Gutierrez FISICOQUIMICA 3 AUXILIAR DE DOCENCIA: Univ. Miguel Angel Gutierrez P4(g) PCl5(g) P4O10(s) PO43-(ac) HPO42-(ac) H2PO4 –(ac) H3PO4(ac) FISICOQUIMICA 128.9 -287 -343 -2940 -1277 -1292.1 -1296.3 -1288.3 72.4 -267.8 -278 -2675 -1018 -1089.3 -1130.4 -1142.7 128.9 311.7 364.5 228.9 -222 -33 90.4 158 249.2 0 143 -229.94 -241.826 -285.840 -187.8 -191.2 279 129 101 0 0.30 41.8 -17.7 -20.2 -39 -296.8 -396 -907.51 -885.75 -813.989 -907.51 231.7 0 163 -157.30 -228.60 -237.192 -120.4 -134.1 239 80.1 49.1 0 0.096 83.7 12.6 -33 -27.4 -300.2 -371 -741.99 -752.87 -690.059 -741.99 160.95 205.0 238.82 -10.54 188.72 69.940 110 144 168 228.1 430.211 31.9 32.6 22 61.1 205.6 122 248.1 256.66 17 126.9 156.90 17 78.9 -255.6 -329.1 0 -273 121.0 -234 -167.46 0 -92.31 -167.46 111.9 -218.9 -120.9 30.91 0 -36 106.8 -194.7 -55.94 61.8 -262.5 -276.5 0 -275 105.0 -240 -131.17 0 -95.30 -131.17 82.40 158.64 145.47 -9.6 202.7 173.67 165.1 153.25 55.10 223.0 186.79 55.06 174.90 -102.82 3.13 0 -53.5 70.21 80.71 245.78 152.23 198.59 180.67 -51.67 109.4 Grupo VIA O(g) O2(g) O3(g) OH-(ac) H2O(g) H2O(l) H2O2(l) H2O2(ac) S(g) S2(g) S8(g) S (rómbico) S(monoclínico) S2-(ac) HS-(ac) H2S-(g) H2S(ac) SO2(g) SO3(g) SO42-(ac) HSO4-(ac) H2SO4(l) H2SO4(ac) Grupo VIIA F(g) F-(g) F-(ac) F2(g) HF(g) Cl(g) Cl-(g) Cl-(ac) Cl2(g) HCl(g) HCl(ac) Br(g) Br -(g) Br -(ac) Br2(g) Br2(l) HBr(g) I(g) I-(g) I-(ac) AUXILIAR DE DOCENCIA: Univ. Miguel Angel Gutierrez FISICOQUIMICA 4 AUXILIAR DE DOCENCIA: Univ. Miguel Angel Gutierrez FISICOQUIMICA 62.442 0 25.9 19.38 0 1.3 260.58 116.14 206.33 51.9 64.39 341.1 0 105.9 289.2 0 -203 -127.03 -99.50 -62.38 -31.8 50.2 64.98 301.4 0 77.111 250.4 0 -185 -109.72 -95.939 -66.32 -40.3 -26 -98.7 166.29 33.1 73.93 172.892 42.702 84 96.11 107.1 114 146 -152.4 130.5 0 -348.0 -203 -72.38 112.8 0 -144 -106.5 160.9 41.6 43.9 57.7 -61.1 167.64 51.5 71 61.30 0 -230 -264.9 -90.79 -147.21 94.93 0 -318.2 -198 -77.74 78.20 0 -141 164.8 153.9 31.8 0 -184 -210.66 -58.50 174.87 76.027 144 196 70.27 -1971 0 -863.2 -1461 0 -706 -1257 23.8 38 214 Mn2+(ac) Mn(s,a) MnO2 (s) MnO4 –(ac) -219 0 -520.9 -518.4 -223 0 -466.1 -425.1 -84 31.8 53.1 190 Grupo VIIIB Fe3+(ac) Fe2+(ac) Fe(s) Fe(l) -47.7 -87.9 0 13.13 -10.5 -84.94 0 11.05 -293 113 27.3 34.29 I2(g) I2(s) HI(g) Grupo IB Cu+(ac) Cu2+(ac) Cu(g) Cu(s) Ag+(ac) Ag(g) Ag(s) AgF(s) AgCl(s) AgBr(s) AgI(s) Ag2S(s) Grupo IIB Zn2+(ac) Zn(g) Zn(s) ZnO(s) ZnS(s, blenda de zinc) Cd2+(ac) Cd(g) Cd(s) CdS(s) Hg2+(ac) Hg22+(ac) Hg(g) Hg(l) HgCl2(s) Hg2Cl2(s) HgO(s) Grupo VIB (Cr ( H2O)6)3+(ac) Cr(s) CrO42-(ac) Cr2O72-(ac) Grupo VIIB AUXILIAR DE DOCENCIA: Univ. Miguel Angel Gutierrez FISICOQUIMICA 5 AUXILIAR DE DOCENCIA: Univ. Miguel Angel Gutierrez -272.0 -825.5 -1121 -67.4 0 -64.0 0 FeO(s) Fe2O3(s) Fe3O4(s) Co2+(ac) Co(s) Ni2+(ac) Ni(s) FISICOQUIMICA -251.4 -743.6 -1018 -51.5 0 -46.4 0 60.75 87.400 145.3 -155 30 -159 30.1 CAPACIDADES CALORÍFICAS MOLARES DE GASES A PRESIÓN CONSTANTE (De acuerdo a la expresión Cp = a + bT cT2 ) (Válidas para temperaturas entre 300 – 1500 K) cal/K-mol H2 O2 N2 Cl2 CO CO2 H2O(v) NH3 SO2 CH4 C2H6 C2H4 Cp Cp Cp Cp Cp Cp Cp Cp Cp Cp Cp Cp = = = = = = = = = = = = 6.947 6.148 6.524 7.576 6.420 6.214 7.256 6.189 6.147 3.381 2.247 2.830 – – – – – – – – – – – – 0.00020T + 0.00310T 0.00125T 0.00242T 0.00167T 0.0104T – 0.00230T + 0.00789T 0.0138T 0.0180T 0.0382T 0.0286T + 0.48 ∗ 10-6 T2 0.92 ∗ 10-6 T2 0.001 ∗ 10-6 T2 0.97 ∗ 10-6 T2 0.196 ∗ 10-6 T2 3.55 ∗ 10-6 T2 0.28 ∗ 10-6 T2 0.73 ∗ 10-6 T2 9.10 ∗ 10-6 T2 4.30 ∗ 10-6 T2 11.05 ∗ 10-6 T2 8.73 ∗ 10-6 T2 PRESIÓN DE VAPOR DEL AGUA Temperatura (oC) Presión (mmHg) Temperatura (oC) Presión (mmHg) 0 5 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 4.6 6.5 9.2 9.8 10.5 11.2 12.0 12.8 13.6 14.5 15.5 16.5 17.5 18.7 19.8 21.1 22.4 23.8 25.2 27 28 29 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100 105 26.7 28.3 30.0 31.8 42.2 55.3 71.9 92.5 118.0 149.4 187.5 233.7 289.7 355.1 433.6 525.8 633.9 760.0 906.1 AUXILIAR DE DOCENCIA: Univ. Miguel Angel Gutierrez FISICOQUIMICA 6
Anuncio
Documentos relacionados
Descargar
Anuncio
Añadir este documento a la recogida (s)
Puede agregar este documento a su colección de estudio (s)
Iniciar sesión Disponible sólo para usuarios autorizadosAñadir a este documento guardado
Puede agregar este documento a su lista guardada
Iniciar sesión Disponible sólo para usuarios autorizados