El átomo
Anuncio

Phys_RCcap11 10/13/05 11:47 PM Page 147 Nombre Clase Fecha Hoja de destrezas Vocabulario y resumen de la sección El átomo VOCABULARIO Define los siguientes términos en tus propias palabras. Usa el espacio en blanco correspondiente. 1. protón 2. unidad de masa atómica 3. neutrón 4. número atómico 5. isótopo 6. número de masa 7. masa atómica Copyright © by Holt, Rinehart and Winston. All rights reserved. HOLT CIENCIAS Y TECNOLOGÍA 147 Introducción a los átomos Phys_RCcap11 10/13/05 11:47 PM Page 148 Nombre Clase Fecha Vocabulario y resumen de la sección (continuación) RESUMEN DE LA SECCIÓN Lee el siguiente resumen de la sección. • Los átomos son extremadamente pequeños. Los objetos de tamaño normal están formados por grandes cantidades de átomos. • Los átomos están formados por un núcleo, que tiene protones y a menudo neutrones, y por electrones, que están en nubes de electrones alrededor del núcleo. • El número de protones del núcleo de un átomo es el número atómico de ese átomo. Todos los átomos de un elemento tienen el mismo número atómico. • Los distintos isótopos de un elemento tienen en su núcleo un número diferente de neutrones. Los isótopos de un elemento comparten la mayoría de las propiedades químicas y físicas. • • El número de masa de un átomo es la suma de los neutrones y los protones del átomo. • Las fuerzas que operan en un átomo son la fuerza gravitacional, la fuerza electromagnética, la fuerza fuerte y la fuerza débil. La masa atómica es un promedio ponderado de la masa de los isótopos naturales de un elemento. Copyright © by Holt, Rinehart and Winston. All rights reserved. HOLT CIENCIAS Y TECNOLOGÍA 148 Introducción a los átomos ANSWER KEY Vocabulary and Section Summary 5. He performed experiments and drew SECTION: DEVELOPMENT OF THE ATOMIC THEORY 6. 1. atom: the smallest unit of an element that maintains the properties of an element 2. electron: a subatomic particle that has a negative charge 3. nucleus: in physical science, an atom’s central region, which is made up of protons and neutrons 4. electron cloud: a region around the nucleus of an atom where electrons are likely to be found 7. 8. SECTION: THE ATOM 1. proton: a subatomic particle that has a 2. 3. 4. 5. 6. 7. 9. positive charge and that is found in the nucleus of an atom atomic mass unit: a unit of mass that describes the mass of an atom or molecule neutron: a subatomic particle that has no charge and that is found in the nucleus of an atom atomic number: the number of protons in the nucleus of an atom; the atomic number is the same for all atoms of an element isotope: an atom that has the same number of protons (or the same atomic number) as other atoms of the same element do but that has a different number of neutrons (and thus a different atomic mass) mass number: the sum of the numbers of protons and neutrons in the nucleus of an atom atomic mass: the mass of an atom expressed in atomic mass units 10. conclusions from them to develop his theory. Rutherford’s gold foil experiment, in which Rutherford observed that most of the positively charged particles that he aimed at a piece of gold foil went straight through Bohr suggested that electrons could move around the nucleus only in certain paths. They could jump from path to path, but not stay between the paths. Bohr’s theory held that electrons can travel only in certain paths around the nucleus. The current atomic theory is that electrons travel in regions where they are likely to be found. Rutherford placed a surface behind the gold foil, which would glow where the positively charged particles hit it. This shows that he was trying to find out where the particles went after hitting the gold foil. The model represents electrons as mixed throughout an atom. Rutherford showed this arrangement to be incorrect. SECTION: THE ATOM 1. Sample answer: Different isotopes 2. 3. 4. 5. Section Review SECTION: DEVELOPMENT OF THE ATOMIC THEORY 6. 1. Sample answer: the smallest part of an 7. element that has the properties of that element 2. electron 3. nucleus 4. B 8. have the same number of protons but different numbers of neutrons. atomic number atomic mass B Gravitational force acts between objects based on their mass. Electromagnetic force attracts objects of opposite electric charge and repels objects of the same electric charge. The strong force holds the protons and neutrons of atomic nuclei together. The weak force plays a role in radioactive decay. (0.30 203 amu) (0.70 205 amu) 204.4 amu Gravitational force in the nucleus is so small because the masses of nuclear particles are so small. no; Without neutrons, two protons brought into close contact would repel each other. Copyright © by Holt, Rinehart and Winston. All rights reserved. Holt Science and Technology 62 Introduction to Atoms

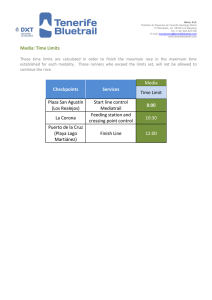




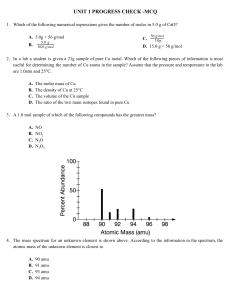
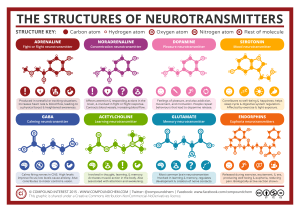





![Chemistry Workbook for Dummies, 3rd Edition (2017) [Unformatted]](http://s2.studylib.es/store/data/009235065_1-1357cc36fbc16624c13a7ec2e0e01f95-300x300.png)