Findings in the Gerstmann–Sträussler–Scheinker
Anuncio
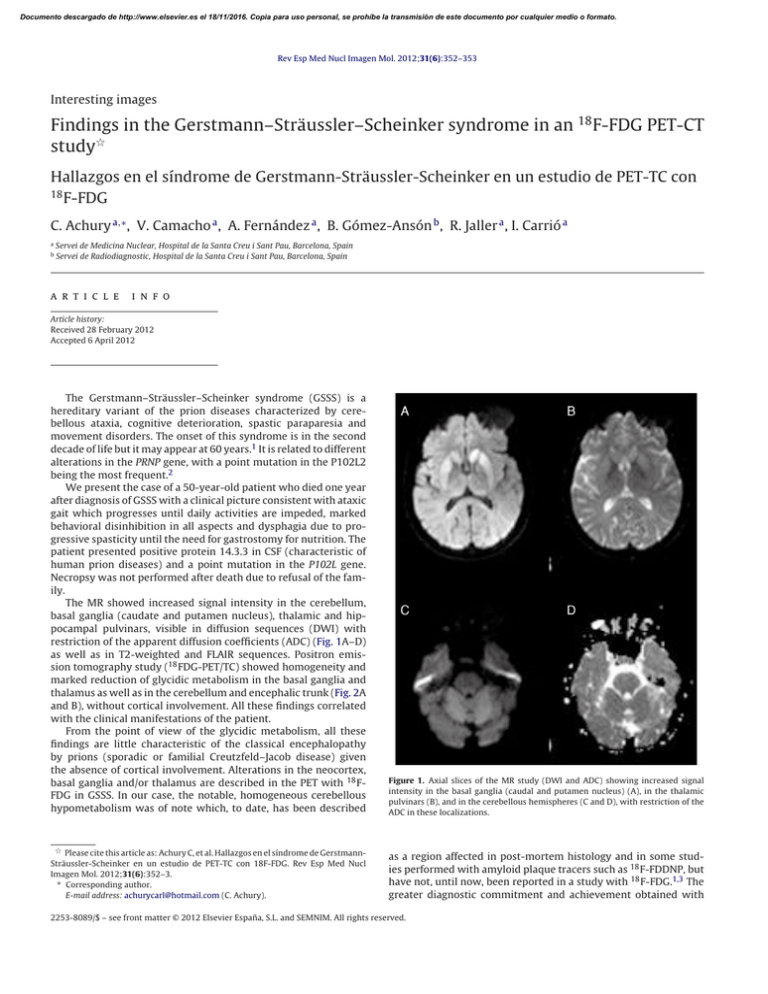
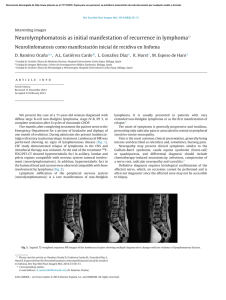
Documento descargado de http://www.elsevier.es el 18/11/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato. Rev Esp Med Nucl Imagen Mol. 2012;31(6):352–353 Interesting images Findings in the Gerstmann–Sträussler–Scheinker syndrome in an 18 F-FDG PET-CT study夽 Hallazgos en el síndrome de Gerstmann-Sträussler-Scheinker en un estudio de PET-TC con 18 F-FDG C. Achury a,∗ , V. Camacho a , A. Fernández a , B. Gómez-Ansón b , R. Jaller a , I. Carrió a a b Servei de Medicina Nuclear, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain Servei de Radiodiagnostic, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain a r t i c l e i n f o Article history: Received 28 February 2012 Accepted 6 April 2012 The Gerstmann–Sträussler–Scheinker syndrome (GSSS) is a hereditary variant of the prion diseases characterized by cerebellous ataxia, cognitive deterioration, spastic paraparesia and movement disorders. The onset of this syndrome is in the second decade of life but it may appear at 60 years.1 It is related to different alterations in the PRNP gene, with a point mutation in the P102L2 being the most frequent.2 We present the case of a 50-year-old patient who died one year after diagnosis of GSSS with a clinical picture consistent with ataxic gait which progresses until daily activities are impeded, marked behavioral disinhibition in all aspects and dysphagia due to progressive spasticity until the need for gastrostomy for nutrition. The patient presented positive protein 14.3.3 in CSF (characteristic of human prion diseases) and a point mutation in the P102L gene. Necropsy was not performed after death due to refusal of the family. The MR showed increased signal intensity in the cerebellum, basal ganglia (caudate and putamen nucleus), thalamic and hippocampal pulvinars, visible in diffusion sequences (DWI) with restriction of the apparent diffusion coefficients (ADC) (Fig. 1A–D) as well as in T2-weighted and FLAIR sequences. Positron emission tomography study (18 FDG-PET/TC) showed homogeneity and marked reduction of glycidic metabolism in the basal ganglia and thalamus as well as in the cerebellum and encephalic trunk (Fig. 2A and B), without cortical involvement. All these findings correlated with the clinical manifestations of the patient. From the point of view of the glycidic metabolism, all these findings are little characteristic of the classical encephalopathy by prions (sporadic or familial Creutzfeld–Jacob disease) given the absence of cortical involvement. Alterations in the neocortex, basal ganglia and/or thalamus are described in the PET with 18 FFDG in GSSS. In our case, the notable, homogeneous cerebellous hypometabolism was of note which, to date, has been described Figure 1. Axial slices of the MR study (DWI and ADC) showing increased signal intensity in the basal ganglia (caudal and putamen nucleus) (A), in the thalamic pulvinars (B), and in the cerebellous hemispheres (C and D), with restriction of the ADC in these localizations. 夽 Please cite this article as: Achury C, et al. Hallazgos en el síndrome de GerstmannSträussler-Scheinker en un estudio de PET-TC con 18F-FDG. Rev Esp Med Nucl Imagen Mol. 2012;31(6):352–3. ∗ Corresponding author. E-mail address: [email protected] (C. Achury). as a region affected in post-mortem histology and in some studies performed with amyloid plaque tracers such as 18 F-FDDNP, but have not, until now, been reported in a study with 18 F-FDG.1,3 The greater diagnostic commitment and achievement obtained with 2253-8089/$ – see front matter © 2012 Elsevier España, S.L. and SEMNIM. All rights reserved. Documento descargado de http://www.elsevier.es el 18/11/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato. C. Achury et al. / Rev Esp Med Nucl Imagen Mol. 2012;31(6):352–353 353 PET/CT with 18 F-FDG compared to images obtained with MR is of note.3 Although there is currently no specific treatment for this encephalopathy and the outcome is always fatal, early diagnosis such as that which may be obtained with a PET/CT study with 18 FFDG may provide early accompaniment and family support in the management of this disease. Bibliografía 1. Collins S, McLean CA, Masters CL. Gerstmann–Sträussler–Scheinker syndrome, fatal familial insomnia, and kuru: a review of these less common human transmissible spongiform encephalopathies. J Clin Neurosci. 2011;8:387–97. 2. Ortega-Cubero S, Luquín MR, Domínguez I, Arbizu J, Pagola I, Carmona-Abellán MM, et al. Neuroimagen estructural y funcional en las enfermedades priónicas humanas. Neurología. 2011, http://dx.doi.org/10.1016/j.nrl.2011.03.012. 3. Kepe V, Ghetti B, Martin R, Farlow MR, Bresjanac M, Miller K, et al. PET of brain prion protein amyloid in Gerstmann–Sträussler–Scheinker disease. Brain Pathol. 2010;20:419–30. Figure 2. PET/CT study with 18 F-FDG (axial slices) showing severe decrease of glycidic metabolism in both basal ganglia and both thalamus (A) as well as marked hypometabolism in the cerebellum and encephalic trunk (B).