Document
Anuncio

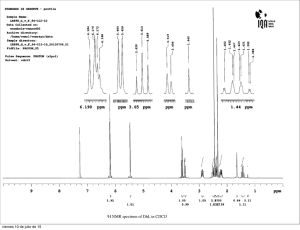
Supporting Information Antileishmanial Metabolites from Geosmithia langdonii Lourin G. Malak,†,‡ Mohamed Ali Ibrahim,†,§ Daoud W. Bishay,‡ Afaf M. Abdelbaky,‡ Ahmed M. Moharram, Babu Tekwani,† Stephen J. Cutler,║ and Samir A. Ross†,,* †National Center for Natural Products Research, School of Pharmacy, The University of Mississippi, University, MS 38677, USA ‡Pharmacognosy Department, Faculty of Pharmacy, Assiut University, Assiut 71526, Egypt §Department of Chemistry of Natural Compounds, National Research Center, Dokki 12622, Cairo, Egypt Assiut University Mycological Center, Assiut University, Assiut 71515, Egypt ║Medicinal Chemistry Department, School of Pharmacy, University of Mississippi, University, MS 38677, USA Pharmacognosy Department, School of Pharmacy, University of Mississippi, University, MS 38677, USA Table of contents: Fig. Title Page SI 1 1H NMR spectrum of 4-(2`,4`-dihydroxy-6`-(hydroxymethyl)benzyl) benzene-1,2-diol (1) [400 MHz, CD3OD] 5 SI 2 13C NMR spectrum of 4-(2`,4`-dihydroxy-6`-(hydroxymethyl) benzyl) benzene-1,2-diol (1) [100 MHz, CD3OD] 6 SI 3 DEPT spectrum of 4-(2`,4`-dihydroxy-6`- (hydroxymethyl) benzyl) benzene-1,2-diol (1) [CD3OD] 7 SI 4 HMQC spectrum of 4-(2`,4`-dihydroxy-6`-(hydroxymethyl) benzyl) benzene-1,2-diol (1) [CD3OD] 8 SI 5 HMBC spectrum of 4-(2`,4`-dihydroxy-6`-(hydroxymethyl) benzyl) benzene-1,2-diol (1) [CD3OD] 9 SI 6 1H SI 7 13C NMR spectrum of (4R, 5R, 6R)-4,5-dihydroxy-6-(6`-methylsalicyloxy) -2-methyl-2-cyclohexen-1-one (2) [100 MHz, pyridine-d5] 11 SI 8 DEPT spectrum of (4R, 5R, 6R)-4,5-dihydroxy-6-(6`-methylsalicyloxy) -2methyl-2-cyclohexen-1-one (2) [pyridine-d5] 12 SI 9 HMQC spectrum of (4R, 5R, 6R)-4,5-dihydroxy-6-(6`-methylsalicyloxy) - 13 2-methyl-2-cyclohexen-1-one (2) [pyridine-d5] NMR spectrum of (4R, 5R, 6R)-4,5-dihydroxy-6-(6`-methylsalicyloxy) - 10 2-methyl-2-cyclohexen-1-one (2) [400 MHz, pyridine-d5] Fig. Title Page SI 10 HMBC spectrum of (4R, 5R, 6R)-4,5-dihydroxy-6-(6`-methylsalicyloxy) -2- 14 methyl-2-cyclohexen-1-one (2) [pyridine-d5] SI 11 Reaction of compound 2 with either (R)- or (S)-MTPA 15-18 SI 12 NOESY spectrum of (4R, 5R, 6R)-4,5-dihydroxy-6-(6`-methylsalicyloxy) 2-methyl-2-cyclohexen-1-one (2) [400 MHz, pyridine] 19 SI 13 1H 20 SI 14 13C SI 15 Reaction of compound 3 with either (R)- or (S)-MTPA 22-25 SI 16 1H 26 SI 17 13C SI 18 NMR spectrum (+)-epiepoformin (3) [400 MHz, CDCl3] NMR spectrum of (+)-epiepoformin (3) [100 MHz, CDCl3] NMR spectrum of (-) dihydroepiepoformin (4) [400 MHz, CDCl3] 21 NMR spectrum of (-) dihydroepiepoformin (4) [100 MHz, CDCl3] 27 1H NMR spectrum of (4S,5S)-4,5-dihydroxy-2-methyl-cyclohex-2-enone (5) [400 MHz, CD3OD] 28 SI 19 13C NMR spectrum of (4S,5S)-4,5-dihydroxy-2-methyl-cyclohex-2-enone (5) [100 MHz, CD3OD] 29 SI 20 1H 30 SI 21 13C NMR spectrum of 6-methylsalicylic acid (6) [400 MHz, pyridine-d5] NMR spectrum of 6-methylsalicylic acid (6) [100 MHz, pyridine-d5] 31 Fig. Title Page SI 22 1H SI 23 13C SI 24 1H SI 25 13C SI 26 1H NMR spectrum of 2,5-dihydroxy-benzaldehyde (9) [400 MHz, CD3OD] 36 SI 27 13C 37 SI 28 13C NMR spectrum of 2,5-dihydroxy-benzaldehyde (9) [100 MHz, CD3OD] 38 SI 29 1H 39 SI 30 13C SI 31 1H NMR spectrum of 2,5-dihydroxybenzyl alcohol (11) [400 MHz, CD3OD] 41 SI 32 13C 42 SI 33 1H 43 SI 34 13C SI35 Selected HMBC correlations for compounds 1 and 2 NMR spectrum of gentisylquinone (7) [400 MHz, CDCl3] 32 NMR spectrum of gentisylquinone (7) [100 MHz, CDCl3] 33 NMR spectrum of 3,4-dihydroxytoluene (8) [400 MHz, CD3OD] 34 NMR spectrum of 3,4-dihydroxytoluene (8) [100 MHz, CD3OD] NMR spectrum of 2,5-dihydroxy-benzaldehyde (9) [100 MHz, CDCl3] NMR spectrum of 3-hydroxybenzyl alcohol (10) [400 MHz, CD3OD] NMR spectrum of 3-hydroxybenzyl alcohol (10) [100 MHz, CD3OD] NMR spectrum of 2,5-dihydroxybenzyl alcohol (11) [100 MHz, CD3OD] NMR spectrum of 3-hydroxytoluene (12) [400 MHz, pyridine-d5] NMR spectrum of 3-hydroxytoluene (12) [100 MHz, pyridine-d5] 35 40 44 45 SI 1: 1H NMR spectrum of 4-(2`,4`-dihydroxy-6`-(hydroxymethyl) benzyl) benzene-1,2-diol (1) [400 MHz, CD3OD] SI 2: 13C NMR spectrum of 4-(2`,4`-dihydroxy-6`-(hydroxymethyl) benzyl) benzene-1,2-diol (1) [100 MHz, CD3OD] SI 3: DEPT spectrum of 4-(2`,4`-dihydroxy-6`- (hydroxymethyl) benzyl) benzene-1,2-diol (1) [CD3OD] SI 4: HMQC spectrum of 4-(2`,4`-dihydroxy-6`(hydroxymethyl)benzyl)benzene-1,2-diol (1) [CD3OD] SI 5: HMBC spectrum of 4-(2`,4`-dihydroxy-6`-(hydroxymethyl) benzyl) benzene-1,2-diol (1) [CD3OD] SI 6: 1H NMR spectrum of (4R, 5R, 6R)-4,5-dihydroxy-6-(6`methylsalicyloxy) -2-methyl-2-cyclohexen-1-one (2) [400 MHz, pyridine-d5] SI 7: 13C NMR spectrum of (4R, 5R, 6R)-4,5-dihydroxy-6-(6`methylsalicyloxy) -2-methyl-2-cyclohexen-1-one (2) [100 MHz, pyridine-d5] SI 8: DEPT spectrum of (4R, 5R, 6R)-4,5-dihydroxy-6-(6`-methylsalicyloxy) -2-methyl-2-cyclohexen-1-one (2) [pyridine-d5] SI 9: HMQC spectrum of (4R, 5R, 6R)-4,5-dihydroxy-6-(6`-methylsalicyloxy) -2-methyl-2-cyclohexen-1-one (2) [pyridine-d5] SI 10: HMBC spectrum of (4R, 5R, 6R)-4,5-dihydroxy-6-(6`methylsalicyloxy) -2-methyl-2-cyclohexen-1-one (2) [pyridine-d5] SI 11: Reaction of compound 2 with either (R)- or (S)-MTPA Mosher esters formation of compound 2 Compound 2 + R-MTAP H-3 H-6 H-5 H-4 H-4 Compound 2 + S-MTAP Final Structure (4R, 5R, 6R)-4,5-dihydroxy-6-(6`-methylsalicyloxy) -2-methyl-2-cyclohexen-1-one Final Structure Based on NOE SI 12: NOESY spectrum of (4R, 5R, 6R)-4,5-dihydroxy-6-(6`methylsalicyloxy) -2-methyl-2-cyclohexen-1-one (2) [pyridine-d5] SI 13: 1H NMR spectrum (+)-epiepoformin (3) [400 MHz, CDCl3] SI 14: 13C NMR spectrum of (+)-epiepoformin (3) [100 MHz, CDCl3] SI 15: Reaction of compound 3 with either (R)- or (S)-MTPA Mosher esters formation of compound 3 Compound 3 + SMTPA / Pyridine-d5 Compound 3 + RMTPA / Pyridine-d5 Compound 3 / Pyridine-d5 Compound 3 + SMTPA / Pyridine-d5 Compound 3 + RMTPA / Pyridine-d5 Final Structure of Compound 3 SI 16: 1H NMR spectrum of (-) dihydroepiepoformin (4) [400 MHz, CDCl3] SI 17: 13C NMR spectrum of (-) dihydroepiepoformin (4) [100 MHz, CDCl3] SI 18: 1H NMR spectrum of (4S,5S)-4,5-dihydroxy-2-methyl-cyclohex-2enone (5) [400 MHz, CD3OD] SI 19: 13C NMR spectrum of (4S,5S)-4,5-dihydroxy-2-methyl-cyclohex-2enone (5) [100 MHz, CD3OD] SI 20: 1H NMR spectrum of 6-methylsalicylic acid (6) [400 MHz, pyridine-d5] SI 21: 13C NMR spectrum of 6-methylsalicylic acid (6) [100 MHz, pyridine-d5] SI 22: 1H NMR spectrum of gentisylquinone (7) [400 MHz, CDCl3] SI 23: 13C NMR spectrum of gentisylquinone (7) [100 MHz, CDCl3] SI 24: 1H NMR spectrum of 3,4-dihydroxytoluene (8) [400 MHz, CD3OD] SI 25: 13C NMR spectrum of 3,4-dihydroxytoluene (8) [100 MHz, CD3OD] SI 246: 1H NMR spectrum of 2,5-dihydroxy-benzaldehyde (9) [400 MHz, CD3OD] SI 27: 13C NMR spectrum of 2,5-dihydroxy-benzaldehyde (9) [100 MHz, CDCl3] SI 28: 13C NMR spectrum of 2,5-dihydroxy-benzaldehyde (9) [100 MHz, CD3OD] SI 29: 1H NMR spectrum of 3-hydroxybenzyl alcohol (10) [400 MHz, CD3OD] SI 30: 13C NMR spectrum of 3-hydroxybenzyl alcohol (10) [100 MHz, CD3OD] SI 31: 1H NMR spectrum of 2,5-dihydroxybenzyl alcohol (11) [400 MHz, CD3OD] SI 32: 13C NMR spectrum of 2,5-dihydroxybenzyl alcohol (11) [100 MHz, CD3OD] SI 33: 1H NMR spectrum of 3-hydroxytoluene (12) [400 MHz, pyridine-d5] x SI 34: 13C NMR spectrum of 3-hydroxytoluene (12) [100 MHz, pyridine-d5] SI 35: Selected HMBC correlations for compounds 1 and 2