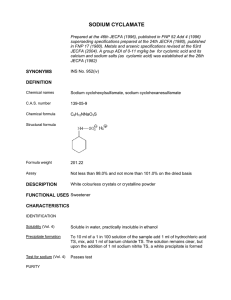
2002 SME Annual Meeting Feb. 25 - 27, Phoenix, Arizona Preprint 02-160 ENHANCED SLURRY WETTING AND DISPERSION WITH SODIUM SILICATE: AN EFFECTIVE ROUTE TO OPTIMIZING SULFIDE PROCESSING D.R. Shaw Atwood, KS R. Reifsnyder and J. LaRosa Thompson PQ Corp Valley Forge, PA ABSTRACT A key component of mineral processing operations, particularly of flotation, concerns the use of surface active compounds designed to absorb at one or more of the interfaces present in the system, thus altering the corresponding interfacial tension. A critical feature of surface chemistry is the wetting or non-wetting of the solid surfaces. Generally, wetting means that the contact angle between a liquid and solid is zero or so close to zero that the liquid spreads over the solid easily, whereas, non0 wetting means that the angle is greater than 90 so that the liquid tends to ball up and run off the surface easily (Adamson, 1976). The wetting action generally is accomplished by the use of surfactant additives that consist of polar-nonpolar type molecules. The non-polar portion usually is a hydrocarbon, aliphatic or aromatic in nature. The polar portion may be ionic or nonionic and contain functional groups such as carboxylic acids, esters, ethers, alcohols, in addition to sulfur compounds, sulfonic acids, sulfates, and others containing phosphorus, nitrogen, and halides. Oxygen is a key component of sulfide surface wetting and in the electrochemical reactions with thiol-type collection agents. Although in the case of sulfide flotation, only 5 to 15% of a complete monolayer of adsorbed collection is necessary for flotation, it is nonetheless essential for selective hydrophobicity that as much as possible of the mineral surface be exposed for interactions with reagents such as collectors, frothers, and regulators. This also holds true for other unit operations; for example good surface wetting favors efficient comminution, and liquid expulsion is important in dewatering. Efficient surface wetting is also a key factor in the mechanisms involved with reagents that control the dispersion and aggregation or rheological behaviors of mineral suspensions. It is in this regard that the most practical benefits of good surface wetting may be realized. The mechanisms of several inorganic and organic electrolytes as grinding aids, for example, are well known. Investigations have focused on inorganic additives such as sodium silicate, sodium chloride, ferric chloride, etc., and organic compounds such as sodium tripolyphosphate, alkaliamines, polyelectrolytes, etc. (Klimpel and Manfroy, 1978). The majority of the studies, however, do not consider the mechanisms involved with efficient surface wetting in relation to pulp rheology or flotation behavior (Tucker, 1982). The impact of the liquid electrolyte composition may also be an important factor in surface wetting. The reactions of certain common alkali or pH regulators, such as lime, sodium carbonate, etc, often are not considered in relation to the action of surfactants as regards surface wettability. Consequently, most industrial sulfide flotation plants do not consider the role of surface wetting of the slurries in the context of the highly interrelated unit operations of grinding, flotation, and dewatering. Slurry or slimes dispersion is a critical factor controlling the efficiency of sulfide mineral comminution and concentration operations. The PQ Corporation has conducted innovative sodium silicate-based studies to determine the effects of enhanced dispersion on whole ore grinding and flotation behaviors and on downstream separation and cleaning steps. The work has advanced the understanding of the role of pulp rheology on the optimization of commercial grinding, agitation leaching, and flotation operations. This paper reviews the role of sodium silicate as a selective wetting agent and surface charge modifier in sulfide mineral systems, and discusses the results of silicate experiments conducted on various ores of copper and molybdenum, gold, platinum-palladium, and copper zinc, all of which contain clay minerals or highly floatable gangue constituents. INTRODUCTION PQ Corporation, the world leader in manufacturing and technology development of silicate chemicals, began an investigation in 1997 of new applications of sodium silicate chemicals in minerals processing. The project focused on sulfide ore processing, and silicate was evaluated in both grinding and flotation operations in laboratory and industrial plant tests. Although high-level additions of silicate are known to cause large bulk solution changes, the present investigation centered on lowlevel silicate additions in an attempt to determine the influence of silicate on mineral surface properties, especially as regards particle dispersion. The impact on slurry rheology due to lowlevel silicate-induced dispersion may be significant in terms of optimizing concentrator unit operations of comminution and flotation. The release of polyvalent cations and surface-active silicate ions may also offer certain synergistic effects when silicate is used in connection with other modifiers such as soluble cellulose. Sodium silicate, also known as water glass, has a nearly100-year history in non-metallic minerals processing, being best known, for example, as a depressant agent for quartz and silicate minerals and some types of salt minerals such as calcite, fluorite, and barite, depending on system pH and the presence of polyvalent cations of aluminum, chromium, and iron. Silicate is a well known quartz depressant in the flotation of kaolinite, muriate of potash (sylvinite), and phosphate, as well as in non-ferrous metallic-oxide systems such as chromium, tin, tungsten, etc. Silicate also is used in some iron ore processing, for example, as a dispersant in non-magnetic taconite ore desliming. In sulfide ore treatment, silicate use has been limited primarily to molybdenite ore treatment and down-stream separations, and in certain polymetallic sulfide ore separations. 1 Copyright 2002 by SME 2002 SME Annual Meeting Feb. 25 - 27, Phoenix, Arizona Sodium Silicate As A Wetting And Dispersing Agent Table 1 Type N Silicate Specification %Na2O: Viscosity: Weight ratio, 8.9 180 cp SiO2/Na2O: 3.22 o %SiO2: Density (20 C): pH: 11.3 3 1.38 g/cm 28.7 Sodium silicate is a common additive for dispersing colloidal particles produced during wet grinding. Due to the high surface areas and energies of finely ground particles, coagulation of fine-grained particles to themselves and to coarser-sized particles is common. Aggregation may also occur due to hydrophobic-bonding effects, to liquid-phase electrolyte effects, and to the effects of pH regulators. The hydrolysis of alkaline silicate solutions gives rise to polyvalent polymeric species, which have useful, multifunctional, wetting and dispersing regulating properties in flotation. Several investigators have suggested the presence in solution of monomeric (charged and uncharged) species, dimeric and tetrameric species, as well as polymerized polyvalent aggregates of silicic micelles (Vail, 1952) (Lagerstrom, 1959) (Aveston, 1965). Those species are highly charged and highly reactive with the polyvalent cations existing at the mineral surface and in solution. Such polyvalent polymers would create large forces on all oppositely charged solid surfaces, resulting in rapid charge reversal and highly negative zeta potential. The resulting particle repulsion is believed to be electrostatic in nature, although there may be several bonding forces involved with polymer adsorption as summarized in Attia and Fuerstenau (Attia and Fuerstenau, 1978). Velemakanni and Fuerstenau have discussed the concept of steric statibization, in which the electrical double layer repulsion is the principal factor that controls the dispersion stability of a suspension (Velamakanni and Fuerstenau, 1993). The depolymerization of silicate solutions is dependent on the solution pH and electrolyte composition, and it is likely that the proportion of polymeric species is also pH dependent (Leja, 1982). The aggregates of silicic acid and the electrostaticallyinduced repulsive forces cause dispersion of highly charged particles, such as clay minerals and sulfides, from mineral grains, thus rendering cleaner surfaces to aid in comminution efficiency and collector adsorption or depression by other regulating agents such as lime, sulfite ion, etc. Silica micelles are favored at lower solution pH values, and these species may be important in lower pH flotation systems. The addition of polyvalent cations of aluminum, chromium, iron and others often is necessary to help distinguish the surface charges of minerals such as salt types, which have similar surface properties. Sodium silicate may have some interesting synergistic effects when used together with agents such as soluble cellulose or guar gum in the depression of highly floatable phyllosilicate minerals. An investigation of the cellulose depressant properties at the talc-water interface concluded that the adsorption of carboxymethyl cellulose was enhanced with either increased ionic strength of the aqueous phase or reduced pH (Morris, Fornasiero, and Ralston, 1996). The ionic strength was increased by magnesium ion addition. In the work described herein, the addition of silicate ions may have increased the slurry ionic strength and possibly influenced the levels of talc mineral wetting to the extent that CMC adsorption was stronger. Total Solids: 37.6% Character: Syrupy liquid DISCUSSION OF RESEARCH TRIALS WITH SODIUM SILICATE The following discusses the results of laboratory and plant tests of sodium silicate of several sulfide ore types. Flotation Platinum Group Metals-Sulfide Ore. The results are shown below of testing of silicate added to the SAG mill of a PGM sulfide concentrator. The silicate dosage was approximately 0.16g (as received reagent)/t of ore and was added prior to separate conditioning of the normally used carboxymethyl cellulose (CMC). CMC Reduction With Silicate CMC Addition, % of Normal 100 90 No Silicate 80 70 Silicate 60 -5 -4 -3 -2 -1 0 1 2 3 4 5 6 7 Test Days Figure 1 CMC Reduction With Silicate Concentrate Grade With Silicate Concentrate Grade, Pt+Pd, oz/ton 70 60 50 40 30 No Silicate 20 Silicate 10 0 -4 -3 -2 -1 0 1 2 3 4 5 6 7 Test Days Figure 2 Concentrate Grades With Silicate Sodium Silicate Reagent Essentially all of the concentrate streams (i.e., flash, rougher, scavenger, and cleaner concentrates) were significantly higher, by approximately 30 %, in PGM content when sodium silicate was used together with CMC, than without silicate. PGM recoveries also were slightly higher with silicate. Of particular significance was that these results with silicate occurred at CMC dosages that were reduced by some 30% from the normal level. Thus, in addition to better flotation selectivity, the use of silicate in connection with CMC would result in a significantly lower depressant reagent cost burden. Copper Ore Extensive trials of silicate were carried out at a large capacity copper concentrator in Arizona. The silicate was added to the ball mills at a dosage of 0.13g/t of ore. A summary of the research results follows. 1. Overall, there was an approximately 1.2 to 1.6% increase in copper recoveries in the silicate tests versus the typical copper recovery. Separate laboratory tests indicated a similar (approximately 2.8%) increase in Aside from mineral-related uses, PQ Corporation sodium and potassium silicates have broad industrial applications in areas which include: adhesives and binders, cements/coatings, corrosion inhibitors, detergents, soil stabilizations and grouting, metal fabrication and processing, pulp/paper processing, petroleum recovery, textile processing, deinking, and waste disposal and water treatment. There are several types of commercial silicate solutions, varying by SiO2: Na2O ratio, density, and viscosity. Type N solution, as manufactured by PQ Corporation in the U.S. and its Canadian subsidiary, National Silicates, is widely used in materials processing applications and was used exclusively in the work herein. Type N has a high functional group (i.e., % SiO2) content, relatively low viscosity, and has a relatively low commercial cost. Type N has the following specifications: 2 Copyright 2002 by SME 2002 SME Annual Meeting Feb. 25 - 27, Phoenix, Arizona copper recoveries with silicate. Copper recoveries by particle size were as follows common effect in zinc flotation for a certain amount of carrier or “piggyback” gangue flotation to occur due to lime-induced coagulation or hydrophobic bonding effects. If the dispersing effects of silicate cause more rapid gangue drainage, the flotation rates of zinc are essentially higher. Gold Ore Plant Test A long-term trial of silicate was conducted at a concentrator in Nevada. Although no data are available for publishing at this time, the operation reports significant improvements in metallurgical performance from most of the clay-rich ore types being processed. The operation also reported that the excessive, poorly-draining froth behavior characteristic of clay-rich ores was improved considerably with silicate. The preferred silicate dosage was approximately 0.05 kg/t of ore to the SAG mill. This operation has continued to use silicate and upgraded its delivery system in 2001. Molybdenum Ore Sodium silicate was evaluated in laboratory trials, in the by-product molybdenum cleaning section. Silicate was stage added in the cleaning steps to aid the rejection of insoluble gangue. The results, Figure 4, showed a substantial reduction of the insoluble content of the molybdenum concentrate from approximately 25% without silicate to typically less than 510% with silicate. The molybdenum grade/recovery relationship also improved considerable due to the higher flotation selectivity with silicate. Table 2 Silicate Tests-Copper Recoveries By Size Size Fraction, Copper Copper mm Recoveries, % Recoveries, % By Fraction, With By Fraction, Silicate No Silicate +0.297 60.2 53.8 +0.208 78.8 76.4 +0.150 90.0 88.1 +0.074 96.6 92.8 +0.037 96.2 95.0 -0.037 91.3 91.2 Overall 92.6 89.8 Recoveries increased from most of the size fractions, particularly at the coarser range. 2. Although there were no significant concentrate grade increases with silicate, the rougher and cleaner concentrate grades during long term silicate testing were significantly less variable than without silicate, in which case grade fluctuations were as much as +50 to+100% from day to day. 3. There appeared to be a trend towards more consistent copper recoveries regardless of fluctuations of plant feed rate and copper head assays. Without silicate, there were marked copper recovery reductions at higher feed throughputs and at lower feed grades. 4. Separate testing of silicate added to the cleaner/recleaner feeds resulted in copper recovery increases from approximately 60% to over 80% (cleaner feed basis) with silicate. Concentrate grades also were much steadier with silicate. Insol Rejection with Sodium Silicate Insol 30 20 10 0 0 1 Zn Recovery, % 86.2 80.9-82.4 Copper Ore The effect of sodium silicate on grinding product particle size distributions is shown in Figure 5. -9 -8 -7 -6 -5 -4 -3 -2 -1 0 2 4 6 Mill B-5 Mill B-4 Mill B-3 Mill B-2 Mill B-1 Mill A-6 With Sodium Silicate Section-B AVG Mill Num ber 6 Section-A AVG Silicate Mill A-5 8 Mill A-4 9 Mill A-3 10 Without Sodium Silicate Mill A-2 11 35 33 31 29 27 25 23 21 19 17 15 Mill A-1 Percent of +65 mesh fraction Zn Concentrate, % Fe 12 No Silicate Insol Assay, % Grinding Effects Fe Reduction In Zinc Conc. With Silicate 7 5 Figure 4 Effect Of Silicate On Insol Rejection Mill A-7 %Fe 7.68 10.5-11.1 4 Insol Distribution, % Table 3 Silicate Test Results Copper-Zinc Ore Zn Concentrate %Zn 52.3 49.5 3 Silicate, lb/ton Copper-Zinc Ore The results of silicate tests performed in the zinc cleaner circuit at a major copper-zinc concentrator are shown below. Test Silicate No Silicate 2 Figure 5 Product Size Distribution For Ball Mill Products 8 10 12 15 Test Days Silicate was added to the ball mills in the Arizona copper ore trials discussed in the aforementioned section. On average, the grinding product resulting from silicate was 2.1 weight % finer than typically retained on 65 mesh Tyler (0.208mm). It is noteworthy that the finer product occurred at the same time that the plant tonnage throughput was some 4.5% higher than without silicate. Thus, the results indicate that more efficient grinding occurred due to the slurry dispersion imparted by the low level (0.15 g/t) silicate addition to the ball mills. Gold Ore Another test was carried out at another gold mill, in which sodium silicate was added to the ball mill. The results Figure 3 Fe Reduction In Zinc Concentrate With Silicate The significant iron reduction with silicate is thought to be due to rejection of pyrite which is the principal diluent of the concentrate. It was speculated that the dispersion effects, in both the slurry and froth, may have contributed to an increased rate of gangue drainage. The enhanced drainage rate is particularly important in cases such as this example where there is rapid and large mass transport of solids to the froth. Moreover, the increased dispersion due to silicate may have reduced the 3 Copyright 2002 by SME 2002 SME Annual Meeting Feb. 25 - 27, Phoenix, Arizona REFERENCES showed that the grinding product with silicate increased in overall fineness to approximately 90 wt % minus 0.044mm (325 mesh Tyler) from typically 70-74wt%. In this case, the ball mill tonnage was constant and the mill power draw also was constant, even with the finer product sizing. Adamson, A., 1976, “Wetting, Flotation, And Detergency”, rd Physical Chemistry of Surfaces, Ch.XI, 3 Edition, John Wiley and Sons, Inc., Mc, New York, pp 459-489. Aveston, J., 1965, “Hydrolysis of Sodium Silicate”, Ultracentrifugation in Chloride Solutions, J. Chem. Soc., Vol. 12, No. 4, pp. 4444-4448. Attia, Y. A., Fuerstenau, D.W., and Li, No. 1978, Recent Developments in Separation Sciences, CRC Press, Vol. 4, pp. 51-69. Klimpel, Richard R. and Manfroy, Willy, 1978, “Chemical Grinding Aids for Increasing Throughput in the Wet Grinding of Ores”, Industrial engineering Chemical Process Design and Development, Vol. 17, No. 4, pp. 518-523. Lagerstrom, G., 1959, “Equilibrium Studies of Polyanions: The silicate ions in NaCIO4 medium”, Acta Chem. Scand., Vol. 13, p. 722. Leja, J., 1982, Surface Chemistry of Froth Flotation, Chapter 10, University of British Columbia, Plenum Press, New York, pp 611-680. Morris, G.E., Fornasiero, D. and Ralston, J., 1996, “The Surface Properties of Depressants at the Talc-Water Interface”, International Mineral Processing Congress, Ch. 6, San Francisco, CA, Vol. 4, pp. 43-47. Tucker, P., 1982, “Rheological Factors that Affect the Wet Grinding of Ores”, Trans. Inst. Min. Metall., Section C, Mineral Process Extr. Metall., Vol. 91, pp.C117-C122. Vail, James G., 1952, Soluble Silicates: Their Properties and Uses, Reinhold Publishing Corporation, New York, Vol. 1 and Vol. 2. Velamakanni, Bhaskar V. and Fuerstenau, Douglas W., 1993, “The Effect of the Adsorption of Polymeric Additives on the Wet Grinding of Minerals: Mechanisms of Suspension Stabilization”, Powder Technology, Elsevier Sequoia, pp. 19. Dewatering Effects In no trial case did the use of low-level additions of silicate cause adverse effects on product or tailings thickening or filtration. In fact, in one trial, the flotation tailings with silicate thickened to higher terminal densities than in the absence of silicate. In another trial, the whole ore solids sedimentation rate in thickening was considerably higher with silicate than normally obtained. The moisture content of a zinc concentrate filter cake was lower when silicate was used in zinc cleaner flotation than without the dispersant. It was speculated that, during vacuum filtration, the small degree of dispersion of the zinc concentrate slurry caused less retention of water in the floccules. CONCLUSIONS The results of this investigation indicated that the mild dispersion resulting from the low level additions of sodium silicate caused superior performance or optimization of the key unit operations of grinding, flotation, and dewatering. No adverse effects of mild silicate –induced dispersion occurred in any of the trials. The economic value of the metallurgical improvements would exceed the cost of the reagent in practice, and the low reagent operating cost reflects the low level additions used and the relatively low unit cost of silicate. ACKNOWLEDGMENTS The authors acknowledge the PQ Corporation for permission to publish this information and to the many operators who contributed to the various trial efforts. 4 Copyright 2002 by SME