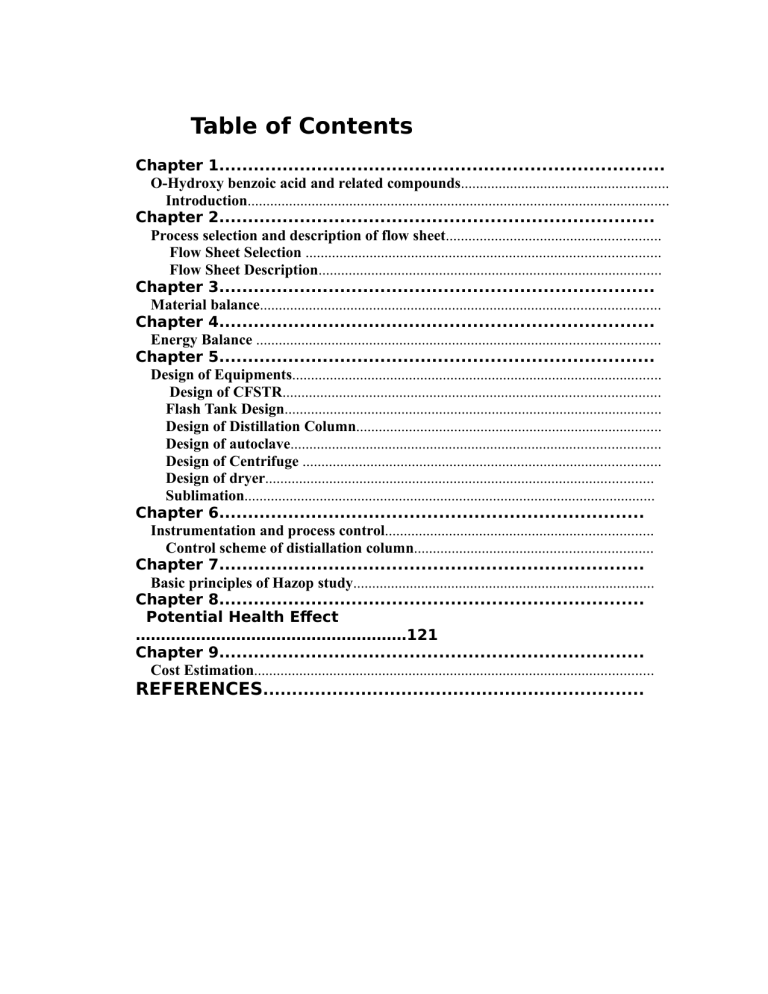
Table of Contents
Chapter 1.............................................................................
O-Hydroxy benzoic acid and related compounds.......................................................
Introduction................................................................................................................
Chapter 2...........................................................................
Process selection and description of flow sheet.........................................................
Flow Sheet Selection ..............................................................................................
Flow Sheet Description...........................................................................................
Chapter 3...........................................................................
Material balance..........................................................................................................
Chapter 4...........................................................................
Energy Balance ...........................................................................................................
Chapter 5...........................................................................
Design of Equipments..................................................................................................
Design of CFSTR....................................................................................................
Flash Tank Design....................................................................................................
Design of Distillation Column.................................................................................
Design of autoclave..................................................................................................
Design of Centrifuge ...............................................................................................
Design of dryer.......................................................................................................
Sublimation.............................................................................................................
Chapter 6.........................................................................
Instrumentation and process control.......................................................................
Control scheme of distiallation column...............................................................
Chapter 7.........................................................................
Basic principles of Hazop study................................................................................
Chapter 8.........................................................................
Potential Health Effect
………………………………………………121
Chapter 9.........................................................................
Cost Estimation..........................................................................................................
REFERENCES..................................................................
2
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Chapter No.1
O-HYDROXY BENZOIC ACID AND RELATED
COMPOUNDS
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Chapter 1
O-HYDROXY BENZOIC ACID AND RELATED
COMPOUNDS
Introduction
Compounds of the general structure
Where the hydroxy is ortho [69-72- 7], meta [99-06.9], or para [9996-7] are commonly known as the monohydroxybenzoic acids. Of the three acids,
the ortho isomer, salicylic acid, is by far the most important. The main importance
of salicylic acid and its derivatives lies in their antipyretic and analgesic actions
(see Analgesics, antipyretics, and anti-inflammatory agents). Natural salicylic
acid, which exists mainly as the glucosides of methyl salicylate [119-38-6] and
salicyl alcohol [90-01-7], is widely distributed in the roots, bark, leaves, and fruits
of various plants and trees. As such, their use as preparations for ancient remedies
is probably as old as herbal therapy. Hippocrates recommended the juice of poplar
trees as treatment for eye diseases. Salicyl alcohol glycosides (salicin) [138-52-3)
occur in Populous halsamifera (poplar) and Snlix helix (willow) trees. Methyl
salicylate glucosides occur in Betula (birch) and Togas (beech) trees. A more
familiar source of methyl salicylate is the leaves of Gaultheria procumbens
(wintergreen) (see also hydroxy carboxylic acids).
Free salicylic acid occurs in nature only in very small amounts. It
has been isolated from the roots, plants, blossoms, and fruit of Spirctea ulmaria,
from which its original name, acidium spiricum, was derived. Salicylic acid as
well as salicyfiltes occur in tulips, hyacinths, and violets, and in common fruits,
eg, oranges, apples, plums, and grapes, which explains the presence of salicylic
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
acid in most wines (1 – 2).
Physical Properties.
Salicylic acid is obtained as white crystals, fine
needles, or fluffy white crystalline powder. It is stable in air and may discolor
gradually in sunlight. The synthetic form is white and odorless. When prepared
from natural methyl salicylate, it may have a lightly yellow or pink tint and a
faint, wintergreenlike odor. Hydroxybenzoic acid crystallizes from water in the
form of white needles and from alcohol as platelets or rhombic prisms. pHydroxybenzoic acid crystallizes in the form of monoclinic prisms. Various
physical properties of hydroxybenzoic acids are listed in Tables 1–4.
Table 1
Physical Properties of Hydroxybenzoic Acids
Property
Value (Isomer)
Ortho
Meta
Para
Molecular weight
138.12
138.12
138.12
Melting point, oC
159
20L5-203
214.5 – 215.6
Boiling point, oC
211 swub
Density
1.443 33
4
1.473 22
29
1.497
Refractive Index
1.565
Flash point (Tag closed-cup), oC
o
20
29
1.57
Ka (acid dissociation) at 25 C
1.05
8.310-5
2.610-5
Heat of combustion, mJ/molo
3.026
3.038
3.035
Heat of sublimation, kJ/molo
95.14
To convert J to cal, divide by 4.184.
116.1
Chapter 1
Table 2
O-Hydroxy Benzoic Acid and Related Compounds
Solubilities of the Hydroxybenzoic Acids in Water, Wt %
Temperature, oC
Isomer
Ortho
Meta
Para
0
0.12
0.35
0.25
10
0.14
0.55
0.50
20
0.20
0.85
0.81
30
0.30
1.35
0.81
40
0.42
2.0
1.23
50
0.64
3.0
2.3
60
0.90
4.3
4.2
70
1.37
7.0
7.0
80
2.21
11.0
12.0
Figure 1. Reactions of the carboxyl group of salicylic acid.
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Reactions. The hydroxybenzoic acids have both the hydroxyl and the
carboxyl moieties and, as such, participate in chemical reactions characteristic of
each. In addition, they can undergo electrophilic ring substitution. Reactions
characteristic of the carboxyl group include decarboxylation; reduction to
alcohols; and the formation of salts, acyl halides, amides, and esters. Reactions
characteristic of the phenolic hydroxyl group include the formation of salts,
esters, and ethers. Reactions involving form sodium salicylate. However, if
salicylic acid dissolves in the presence of alkali metals or caustic alkalies, e.g.,
excess sodium hydroxide, the disodium salt forms.
Salicylic acid can be converted to salicyloyl chloride by reaction with
thionyl chloride in boiling benzene. However, the formation of acyl halides can be
complicated by the presence of the phenolic hydroxyl. For example, the reaction
with phosphorus to arid pentachlorides is not restricted to the formation of the
acid chloride. Further interaction of the phosphorus halide and the phenolic
hydroxyl results in the formation of the phosphoric or phosphorous esters.
The formation of amides can be accomplished by the dehydration of the
ammonium salt of salicylic acid. The more common method for amides is the
reaction of the ester, acylhalide, or anhydride with an amine or ammonia. Each
step is fast and essentially irreversible.
Esterification is frequently carried out by direct reaction of the
carboxylic acid with an alcohol in the presence of a small amount of mineral acid,
usually concentrated sulfuric or hydrochloric acid. The ester of commercial
importance is methyl salicylate. Direct esterification has the advantage of being a
single-step synthesis; its disadvantage is the reversibility of the reaction. The
equilibrium can be shifted to the right if either raw material is used in large
excess, or by selective removal of one of the products. One less frequently
employed technique is the transformation of the acid to the acid chloride followed
by alcoholysis; each step is essentially irreversible. Another method is the reaction
of the alkali salt, eg, sodium salicylate, with an alkyl or an aryl alkyl halide.
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Hydroxyl. The hydroxyl group is alkylated readily by the sodium salt
and an alkyl halide (Williamson ether synthesis) (see Fig. 2). Normally, only Oalkylation is ob ring substitution includes nitration, sulfonation, halogenation,
alkylation, and acylation. The following reactions are illustrated only with
salicylic acid; however, these reactions are characteristic of all the
bydroxybenzoic acids.
Table 3 Solubilities of the Hydroxybenzoic Acids in Non aqueous Solvents,
Wt %
Solvent
Isomer
Ortho
396
0.775
Meta
327
0.010
Para
285
0.0035
28.8 38 c C
20.7 36.5c C
19.5 22.5c C
Ethanol (99 wt %)
40.6 41c C
3.9.6 65c C
38.75 67 c C
n-heptane
2.09 92.2 c C
2.0197 c C
1.5197 c C
o
Acetone at 23 C
Benzene at 25 oC
1-butanol
Methanol at 15 oC
39.87
40.38
36.22
o
Carbon tetrachloride at 25 C
0.262
Chloroform (satd in H2O) at 25 1.84
o
C
Ethanol (abs) at 21 oC
34.87
I-propanol at 21 oC
27.36
Table 4 Saturated Vapor Pressure (p) of o- and p-Hydroxybenzoic Acids
Temperature,
o
C
o-Hydroxybenzoic acid p-Hydroxybenzoic acid
p, Pab
p, Pab
95
30.9
100
48.7
105
70.1
110
104
115
153
120
220
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
125
322
130
3.03
4.59
135
649
6.94
140
10.6
145
16.1
150
23.9
155
34.6
159
47.3
B. To convert Pa to mm Hg, divide by 133.3.
Salicylic Acid
Reactions: Carboxyl. Typical decarboxylation by simple heating of a
free acid occurs with only a few types of acids. However, decarboxylation of
salicylic acid takes place readily because of the presence of the hydroxyl group,
which is electron donating (see Fig. 1). Upon slow heating, salicylic acid
decomposes to phenol and carbon dioxide; when heated rapidly, it sublimes.
Generally, the carboxyl group is not readily reduced. Lithium
aluminum hydride is one of the few reagents that can reduce an acid to an alcohol.
The scheme involves the formation of an alkoxide, which is hydrolyzed to the
alcohol. Commercially, the alternative to direct reduction involves esterification
of the acid followed by reduction of the ester.
Salicylic acid dissolves in aqueous sodium carbonate or ~odium
bicarbonate to serve. However, phenolate ions are ambident nucl~ophiles and, as
such and under certain conditions, as with the use of alkyl halides, the problem of
C- versus O-alkylation can occur. Either reaction can be made essentially
exclusive by the proper choice of reaction conditions. For example, polar solvents
favor formation of the ether, whereas nonpolar solvents favor ring substitution
(see Alkylation).
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Figure 2. Reactions of the hydroxyl group of salicylic acid.
Esters of the phenolic hydroxyl are obtained easily by the SchottenBaumann reaction. The reaction in many cases involves an acid chloride as the
acylating agent. However, acylation can also be achieved by reaction with an acid
anhydride. The single most important commercial reaction of this type is the
acetylation of salicylic acid with acetic anhydride to yield acetylsalicylic acid [5078-2] (aspirin).
Ring Substitution. In the introduction of a third group into a
disnbstituted benzene, the position the group takes depends on the groups present
(see Fig. 3). In the case of salicylic acid the hvdroxyl directs ortho and pan and
the carboxyl directs meta substitution. It is generally accepted that if both an
ortho-para and a meta director are competing for the orientation of a third group,
the ortho-para director prevails since, unlike the meta director, it activates the
ring. Specifically, the hydroxyl group is electron-donating which, on the basis of
resonance considerations, increases the
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Figure 3 Ring-substitution reactions of salicylic acid. X = halogen.
Electron density in the S and 5 positions. The electron-withdrawal
nature of the car-boxyl group decreases the electron density around the 4 and C
positions, which further enhances the electron density of the 3 and 5 positions. As
a rule, direct substitution occurs more easily in the less sterically hindered 5
position, but most often small amounts of the 3 substituted and 3,5-disubstituted
product also form. High yields of the 3-substituted salicylic acid usually can only
be prepared indirectly.
Direct halogenation of salicylic acid is generally carried out in glacial
acetic acid. As expected, the main product is the 5-halo-salicylic acid with small
quantities of the 3-halo- and 3, 5-dihalosalicylic acids.
Reaction with cold nitric acid results primarily in the formation of 5nitrosalicylic acid [96-97-9]. However, reaction with fuming nitric acid results in
decarboxylation as well as the formation of 2, 4, C-trinitrophenol [88-89-1]
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
(picric acid). Sulfonation with chlorosulfonic acid at 16O oC yields 5-sulfosalicylic
acid [56507-30-3]. At higher temperatures (1800 C) and with an excess of
chlorosulfonic acid, 3,5-disulfosalicylic acid forms. Sulfonation with liquid sulfur
trioxide in tetrachloroethylene leads to a nearly quantitative yield of 5sulfosalicylic acid (5).
Because salicylic acid contains the deactivating meta-directing
carboxyl group, Friedel-Crafts reactions (qv) are generally inhibited. This effect is
somewhat offset by the presence of the activating hydroxyl group. Salicylic acid
also reacts with isobutyl or t-butyl alcohol in 80 wt % sulfuric acid at 75 0C to
yield 5-t-butylsalicylic acid [16094-31-8]. In the case of isobutyl alcohol, the
intermediate carbonium ion rearranges to (CH3)3C+.
Miscellaneous. The Reimer-Tiemann reaction of salicylic acid with
chloroform and alkali results in the 3- and 5-formyl derivatives.
If the reaction is carried out with carbon tetrachloride, the
corresponding dicarboxylic acids form.
Alkylation involving formaldehyde in the presence of hydrogen
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
chloride is known as chloromethylation. The reagent may be a mixture of
formalin and hydrochloric acid, paraformaldehyde and hydrochloric acid, a
chloromethyl ether, or a formal. Zinc chloride is commonly employed as a
catalyst, although many others can be used. Chloromethylation of salicylic acid
yields primarily the 5-èubstituted product.
The reaction of salicylic acid with formaldehyde with catalytic amounts
of acid results in the condensation product methylene-5, 5’-disalicylic acid [12225-8].
Salicylic acid, upon reaction with amyl alcohol and sodium, reduces to
a ring-opened aliphatic dicarboxylic acid, ie, pimelic acid. The reaction proceeds
through the intermediate cyclohexanone-2-carboxylic acid.
During certain substitution reactions, the carboxyl group is often
replaced by the entering group. Au example is fuming nitric acid, which results in
the formation of trinitrophenol. Another is the bromination of salicylic acid in
aqueous solution to yield the tribromophenol derivative.
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Salicylic acid couples with diazonium salts in the expected manner.
With diazotized aniline, i.e, benzenediazonium chloride, the primary product is 5phenylazosalicylic acid [314 7-53-3].
The close proximity of the carboxyl and the hydroxyl groups can be
used for beterocyclic synthesis, as in the preparation of hydroxyxanthones (6).
USES
Approximately 60% of the salicylic acid produced in the United States
is consumed in the manufacture of aspirin: this statistic has remained relatively
constant for at least the last ten years. Approximately 10% of the salicylic acid
produced is consumed in various applications, eg, foundry and phenolic resins,
rubber retarders, dyestuffs, and other miscellaneous uses. The remaining 30% is
used in the manufacture of its salts and esters for a variety of applications.
There are many foundry-resin systems in use. Salicylic acid is a small
component only in the Shell process. It is used as a cross-linking agent in the
phenol—fornrnldehyde resin used as a sand core and mold binder and imparts
higher tensile strength. More recent developments have demonstrated that higher
concentrations of salicylic acid than previously used further improve cold and hot
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
tensile strength and reduce cure and machine processing time (18). The
continuing interest in energy and environmental considerations has led to the low
energy processes, which typically do not use salicylic acid. Their growth has been
somewhat limited because of the large capital expenditures required; however, the
economics is expected to shift as the cost of energy increases. Therefore, a zero or
small negative growth for salicylic acid is predicted in foundry-resin applications.
Salicylic acid has also been used in other phenolic resin applications, ie, binders
for grinding wheels, fiber glass, and brake linings (qv) (see Phenolic resins).
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Chapter No. 2
Process Selection and Description
Of Flow Sheet
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Chapter 2
Process Selection and Description of Flow Sheet
FIRST SYNTHESIS
Salicylic acid was first prepared by R.Piria by the fusion of salicyaldehide
with the potassium hydroxide. In 1859, a synthesis method of preparing salicylic
acid was discovered by treating the phenol with carbon dioxide in presence of
metallic sodium. However the only commercial method of manufacturing
salicylic acid until 1874 was the sponification of the methyl salicylate obtained
from the leaves of the winter green or Bark of the birch.
KOLB PROCESS
The first technically suitable process was introduced in 1874 by Kolb. It
involves the reaction of the dry sodium phenolate with carbon dioxide under
pressure and high temperature (180-200) 0C. The drawback of the process was
that the yield was not more than 50% and the separation of byproducts which
were in large quantity was difficult. Salicylic acid is manufactured by the reaction
of phenol with caustic soda and the subsequent treatment of the sodium phenolate
formed with carbon dioxide and acidifying the resultant product with the sulfuric
acid .Phenol and caustic soda are charged in equomolar proportions to a mixer.
The resulting solution is heated to a temperature of 1300C and further evaporated
to dryness in a stirred autoclave or a heated ball mill. Dry carbon dioxide gas is
absorbed and the crude product from the autoclave is dissolved in the equal
amount of water and filtered. The filtrate is precipitated and dried.To obtain pure
product, the crude sodium salicylate solution decolorized with the activated
carbon containing the zinc dust and filtered. The clarified filtrate is acidified with
the excess of sulfuric acid to precipitate the salicylic acid which is centrifuged and
dried to give the high grade salicylic acid.
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
SCHMITT
Schmitt introduced the new lower temperature ranges from 1201400C which significantly increased the yield of the process. The reaction of the
carbon dioxide on the phenol forms an intermediate phenyl carbonate which
rearranges itself to give o-sodium salicylate. The Kolb-Schmitt synthesis method
is still the only industrial process in use in different modifications.
More, et al
More introduced a new step process for the carbonation of dry
sodium phenolate. The reaction is carried out in a stirred reactor. Carbon dioxide
is passed at a temperature of 1300C until 25% of the stiochiometeric amount of
the carbon dioxide is absorbed. In step 11 the temperature of the raised to
2100Cand the remaining carbon dioxide is introduced into the reactor for 5 hr. the
yield of the process was 80 to 82% and for time duration the yield was 76%.
Stopp,et al
The carbonation of the sodium salicylate is carried out in a fluidized bed reactor at
1400C and pressure of 6bar until half of the phenolate is converted to salicylate.
The resulting reaction mixture can be further carbonated in a subsequent stage at a
temperature of 2100Cand a pressure of 10 bars. The subsequent stage may be
fluidized bed reactor or stirred vessel. The yield was 85%.
Barkley et al
The sodium phenolate was cooled to 90 0C. The carbon dioxide
was passed into the autoclave; the temperature was maintained at 120 0C until the
carbon dioxide adsorption was come to an end. The temperature was raised to
induced rearrangement of the intermediate product. The temperature was kept
160-1700Cunder a carbon dioxide pressure 5 bar.
Jenson, et at
An improved method for the production of salicylic acid from phenol
with high degree of conversion and with a significant reduction in the by products
was modified by Jenson and his colleagues. The process comprises of reaction of
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
sodium phenolate with the carbon dioxide indirect single step at a temperature
above 1650C.In the Kolb carboxylation, the reaction between sodium phenolate
with carbon dioxide could advantageously takes place in single step well the
temperature at which sodium phenyl carbonate is ordinarily converted to sodium
salicylate. More particularly instead of introducing carbon dioxide below 150 0C to
produce sodium phenyl carbonate which is then in second step is converted to
sodium salicylate being held above 1650C.
Our contributions
The reaction of sodium phenolate and carbon dioxide is slowest reaction in whole
plant so every scientist focused his attention for finding the set of
thermodynamics properties that would give maximum conversion and minimum
byproduct as well as cost factor would remain under considerations along with the
safe operation.
I was personally interested in finding such data to improve the performance of the
plant used for manufacturing of salicylic acid. The autoclave present in our
department was quite unsuitable for the reaction of carbon dioxide with sodium
phenolate at different temperature and pressure. But fortunately the circumstances
for carrying the experiments in determination the kinetic data I was given
opportunity in the chemistry department lab. I performed number of experiment at
different temperature and different speed of agitation in simple conical flask.
After the result of experiment I decided batch reaction was not economical.
So I made changes in the flow diagram available in literature.
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
FLOW SHEET DESCRIPTION
Preparation of Sodium Phenolate
Phenol is approximately 54% is reacted with
50%caustic soda in a CFSTR. The reaction temperature is 95-990C. The reaction
is exothermic; heat evolved from the reaction is utilized raising the temperature of
the product to 990C. Slightly excess amount of phenol is fed into the reactor
instead of caustic soda because of material of construction is greatly influenced by
the strong alkali. The reaction temperature is so high that lump formation of
sodium phenolate is out of question. Preparation of Sodium salicylate
The product of the first reactor is fed into the flash
tank where separation of water is carries out and then for any batch valve is
opened and autoclave is charged and valve of the carbon dioxide is opened at the
pressure of 8bars for the time of 5-6hrs. The reaction temperature is raised to
about 1250C.
Almost 70% conversion is achieved; carbon dioxide released is recycled back and
then utilized. Now 600Kg of phenol is added so that some sort of azeotrope is
formed and when steam is provided, this mixture is vaporized leaving the thick
slurry of sodium salicylate. The evaporated material is condensed and sent into
the continuous distillation column where separation of phenol and sodium phenate
is carried out.
Acidification tank
The product of the autoclave is thick slurry and it is
diluted with the water in the dilution tank. The solution, so obtained is charged
into the acidification tank where 40% sulfuric acid is reacted that comes from the
storage tank of sulfuric acid. Almost two hr along with agitation gives 100 app.
Conversion. In the acidification tank solid salicylic acid as well as sodium sulfate
is formed
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Purification from water (removal of water)
Removal of water is carried in two steps: in first step
centrifuge removes the large amount of water i.e. from 66 to 16 %water content.
The waste water is sent to the waste water treatment plant. The solid product that
contains salicylic acid and sodium sulfate is sent to the dryer where app. All water
content is removed.
Sublimation and crystallization
The solid product is charged into the sublimation tank
where steam is used as heating medium and vacuum is created with the help of
vacuum pump. At reduced pressure the sublimation of salicylic acid is carried out
at 760C. The vapors coming from the sublimation tank are injected into
crystallizer where condensation of vapors takes place resulting more than 99%
pure product.
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Chapter No. 3
Material Balance
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Chapter 3
Material Balance
Reactor-1
output
input
component
NaOH
H2O
phenol
Na-Ph
total
(Kgmol)
10.65625
66.48056
11.7216
0
88.8584
Kg
426.25
1196.65
1101.83
0
2724.73
%Wt
15.64375
43.91811
40.43814
0
100
Kgmol
0.156
76.98056
1.22
10.5
88.85656
Kg
6.24
1385.65
114.68
1218
2724.57
%Wt
0.22902697
50.8575665
4.20910456
44.704302
100
Kg
6.24
35
101.16
1218
1360.4
%Wt
0.45868862
2.57277271
7.43604822
89.5324904
100
FLASH TANK
input
component
NaOH
H2O
Phenol
Na-Ph
(Kgmol)
0.156
76.98056
1.22
10.5
88.85656
output
Kg
6.24
1385.65
114.68
1218
2724.57
%Wt
0.229027
50.85757
4.209105
44.7043
100
Kgmol
0.156
1.944444
1.07617
10.5
13.67661
side stream
water-out
total
phenol
1350.65
2711.05
13.52
CO2
side stream
231
phenol
Na-Ph
side stream
600
364
Autoclave reactor-2
Addition of CO2 and reaction
input
component
NaOH
H2O
Phenol
Na-Ph
CO2
Na-Sal
(Kgmol)
0.156
1.944444
1.07617
10.5
12.6
output
Kg
%Wt
Kgmol
Kg
%Wt
6.24
0.325951 0.156
6.24
0.36897969
35
1.828249 1.9444
35
2.06959761
101.16
5.284162 1.07617
101.16
5.98172841
1218
63.62307 3.15
365.4
21.6065991
554
28.93857
0
0
0
0
7.35
1183.35
69.9730952
1914.4
100
1691.15
100
Addition of phenol and separation of azeotrope
input
component
NaOH
H2O
Phenol
Na-Ph
CO2
Na-Sal
(Kgmol)
0.156
1.9444
6.395319
3.15
7.35
output
Kg
6.24
35
601.16
365.4
0
1183.35
%Wt
0.284782
1.597335
27.43582
16.67617
0
54.00589
Kgmol
0.156
1.9444
0.01234
0.012069
0
7.35
Kg
6.24
35
1.16
1.4
0
1183.35
%Wt
0.50849529
2.85213707
0.09452797
0.11408548
0
96.4307542
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
2191.15
100
1227.15
100
Kg
6.24
2489.3
1.16
1.4
0
1183.35
3681.45
%Wt
0.16949843
67.617379
0.03150932
0.03802849
0
32.1435847
100
Kg
0
3033.55
1.16
1.4
1014.3
0
541.85
2.45
4594.71
%Wt
0
66.0226652
0.02524642
0.03046982
22.0753867
0
11.7929097
0
0.05332219
100
Kg
0
296.4
0.16
0.4
1014.3
0
539.5
1.225
1851.985
%Wt
0
16.0044493
0.00863938
0.02159845
54.7682622
0
29.1309055
0.06614524
100
side stream
Kgmol
Kg
0
0
152.0639 2737.15
0.01063
1
0.008621 1
0
0
0
0
2.35/142 2.35
0.0125
1.225
2742.725
%Wt
0
99.79673
0.03646
0.03646
0
0
0.085681
0.044664
100
Kg
0
2.96
%Wt
0
0.19008965
side stream
Kgmol
Kg
0
0
16.30222 293.44
%Wt
0
99.53023
Dilution tank
input
component
NaOH
H2O
Phenol
Na-Ph
CO2
Na-Sal
(Kgmol)
0.156
1.9444
0.01234
0.012069
0
7.35
output
Kg
6.24
35
1.16
1.4
0
1183.35
1227.15
%Wt
0.508495
2.852137
0.094528
0.114085
0
96.43075
100
Kgmol
0.156
138.2944
0.01234
0.012069
0
7.35
water
side input
2454.3
Acidification tank
input
component
NaOH
H2O
Phenol
Na-Ph
salicylic acid
Na-Sal
Na2SO4
(Kgmol)
0.156
138.2944
0.01234
0.012069
0
7.35
0
side input
sulfuric acid
water
output
Kg
6.24
2489.3
1.16
1.4
0
1183.35
0
%Wt
0.169498
67.61738
0.031509
0.038028
0
32.14358
0
362.6
543.9
4587.95
Centrifuge
input
component
NaOH
H2O
Phenol
Na-Ph
salicylic acid
Na-Sal
Na2SO4
sulfuric acid
Kgmol
0
168.5306
0.01234
0.012069
7.35
0
3.815845
(Kgmol)
0
168.5306
0.01234
0.012069
7.35
0
3.815845
output
Kg
0
3033.55
1.16
1.4
1014.3
0
541.85
2.45
4594.71
%Wt
0
66.02267
0.025246
0.03047
22.07539
0
11.79291
0.053322
100
Kgmol
0
16.46667
0.001702
0.003448
7.35
0
3.799296
0.0125
Drying unit
input
component
NaOH
H2O
(Kgmol)
0
16.46667
output
Kg
0
296.4
%Wt
0
16.00445
Kgmol
0
0.164444
Chapter 1
Phenol
Na-Ph
salicylic acid
Na-Sal
Na2SO4
sulfuric acid
0.001702
0.003448
7.35
0
3.8
0.0125
O-Hydroxy Benzoic Acid and Related Compounds
0.16
0.4
1014.3
0
539.5
1.225
1851.985
0.008639
0.021598
54.76826
0
29.13091
0.066145
100
0
0.003448
7.35
0
3.8
0
0
0.4
1014.3
0
539.5
0
1557.16
0
0.02568779
65.137815
0
34.6464076
0
100
0.001702
0
0
0
0
0.0125
0.16
0
0
0
0
1.225
294.825
0.054269
0
0
0
0
0.415501
100
Sublimation unit and Crystallization
input
component
NaOH
H2O
Phenol
Na-Ph
salicylic acid
Na-Sal
Na2SO4
sulfuric acid
(Kgmol)
0
0.164444
0
0.003448
7.35
0
3.8
0
output
Kg
0
2.96
0
0.4
1014.3
0
539.5
0
1557.16
%Wt
0
0.19009
0
0.025688
65.13781
0
34.64641
0
100
Kgmol
0
0.069444
0
0
7.333333
0
0
0
Kg
0
1.25
0
0
1012
0
0
0
1013.25
%Wt
0
0.12336541
0
0
99.8766346
0
0
0
100
side stream
Kgmol
Kg
0
0
0.095
1.71
0
0.4
2.3
0
539.5
0
543.91
%Wt
0
0.345829
0
0.080896
0.46515
0
109.1081
0
110
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Chapter No.4
ENERGY BALANCE
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Chapter 4
ENERGY BALANCE FOR THE PROJECT
REACTOR 1 ENERGY BALANE :
NaoH=10.66kgmol
+
Water=
Phenol=11.72kgmol
H2O =
100%
Conversion
at
95 C
Na-phen=
Water =
∆ HR = ∑ Hfp - ∑ H fr
= { 10.656*(-326.6) + 10.656(-258.84)}–{10.656(-165) +
10.656(-426.99)}
∆ HR = 59.247 KJ
TOTAL HEAT RQUIRED = mCp dT + ∆ HR
∆H
= (1.22*220.53KJ/Kgmol.K *
(76.98*75*5)water+(10.5*243.17*5)
0.156*78.62*5 + 59.247 KJ
= 43099 KJ
5)phenol+
+
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
ENERGY BALANCE OF AUTOCLAVE:
CO2=12.6kgmol
30 C
T=102 C
NaOH=0.156kgm
Water = 1.94 kgm
Phenol= 1.07kgm
Na-phen=10.5 //
70 %
conversion
T=125C
NaoH=0.156kgmol
Water=1.944 //
Phenol=1.07 //
Na-phen=3.15 //
Na-sal. = 7.35 //
CO2=5.25 kg
mol
T=125 C
1 ST STEP : SEPARATION OF WATER
TOTAL HEAT RQUIRED = sensible heat + latent heat
=
{ 0.156*78*7}NaOH + { 76.98*75*7}water
+{1.22*298*17}phenol+{
10.5*243*7}Na-ph
+
{1350.65*2551.6}water+
{
13.52/94
*45700
KJ/Kgmol}phenol
= 3513130.53 KJ
nd
2 STEP: ADDITION OF CO2 AND REACTION
HEAT OF REACTION = ∆HR =∑ HFP + ∑HFR
LATENT HEAT REQUIRED = (12.6* 38.65*95)CO2+ 0.156*78*78)NaoH +
(1.944*75*18)H2O + (1.07117*220*18)phenol
+(10.5*243.17*18)Na-ph
= 99310 KJ
TOTAL HEAT RQUIRED = H = Hl + ∆HR = 0
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Hl = ∆HR
Hl = ∑nHfp - ∑nHfr,
∑nHfr + Hl = ∑nHfp
7.35(-393.51* 1000) +7.35(-453*1000) = -7.35* X
X
= 859.511 KJ/gmol
X
= 904.74 KJ/gmol
5% loss so,
3rd step:
addition of phenol and separation of azeotrope
SIMPLE VACUUM SEPARATING THE COTENTS
ENERGY BALANCE OF DILLUTION TANK
Heat balance:
137*75*(T-25)
= {( 0.156*78*(125-T))NaOH +(1.944*75*(125-T))H2O
+(0.01234*220*(125-T))phenol+(7.35*257.76*(125-T))sod.sal
+(0.012069*243*(125-T))Na-phen
Therefore,
1521+18228.75+339.35+236817+3665.96+256875=T{10275–
12.168+145.8+2.7148+1894.
536+2.93)}
T=
62.97 C
ACIDIFICATION TANK
HR =
nHfp - nHfr
{-96909+(9703*44)}*1000 = {3.81158*(-1384.5*1000)KJ+
7.35*(-589.5*1000)}{(-904*7.35)+(-811*3.69)+0.156*(-426)}*1000
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
HR = 93480 KJ
This much amount of heat is contained after the reaction in the system.
Now reaction temp. is 20 C
Heat balance:
Heat input = Heat out put
{3.69*137.777*5}H2SO4 +{ 30.22*75*5}water+ { 0.156*78*(63-20)}NaOH
+{138.2944*75*(63-20)}water + { 0.01234*220*43}phen + {0.0120*69*243.17*(6320)}Na-phen + {7.35*257.76*43}Na-sal
= 542097.75 KJ
This much amount is contained in the system before reaction occurs.
Now the total amount of heat that must be rejected to keep the system at 20C
= 542097.75 + 93480 = 635575.73
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Chapter No. 5
Design of Equipments
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Chapter 5
Design of Equipments
REACTOR DESIGN
Reactor selection
I selected the CSTR
Reactor selection criteria
1- Conversion
2- selectivity
3- productivity
4- safety
5- economics
6- availability
7- flexibility
8- compatibility with processing
9- energy utilization
10- feasibility
11- investment operating cost
12- heat exchange and mixing
Reaction
rate
Volum
e flow
rate
Volum
e of
reactor
Model
Outlet
concen
tration
Inlet
concent
ration
Contacting
pattern
Kinds of impellers
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
A rotating impeller in a fluid imparts flow and shear to it, the shear resulting from
the flow of one portion of the fluid past another. Limiting cases of flow are in the
axial or radial directions so that impellers are classified conveniently according to
which of these flows is dominant. By reason of reflections from vessel surfaces
and
obstruction by affles and other intemals, however, flow patterns in most cases are
mixed. When a close approach to axial flow is particularly desirable, as for
suspension of the solids of a slurry, the impeller may be housed in a draft tube;
and when radial flow is needed, a shrouded turbine consisting of a rotor and a
stator may be employed. Because the performance of a particular shape of
impeller
usually cannot be predicted quantitatively, impeller design is largely an exercise
of judgment so a considerable variety has been put forth by various
manufacturers. A few common types are illustrated on Figure 10.2 and are
described as follows:
a. The three-bladed mixing propeller is modelled on the marine propeller but has
a pitch selected for maximum turbulence. They are used at relatively high speeds
(up to 1800rpm) with low viscosity fluids, up to about 4000cP. Many versions are
available: with cutout or perforated blades for shredding and breaking
up lumps, with sawtooth edges as on Figure 10.2(g) for cutting and tearing action,
and with other than three blades. The stabilizing ring shown in the illustration
sometimes is included to minimize shaft flutter and vibration particularly at low
liquid levels.b. The turbine with flat vertical blades extending to the shaft is
suited to the vast majority of mixing duties up to 100,000 CP or so at high
pumping capacity. The simple geometry of this design and of the turbines of
Figures 10.2(c) and (d) has inspired extensive testing so that prediction of their
performance is on a more rational basis than that of any other kind of impeller.
c. The horizontal plate to which the impeller blades of this turbine are attached
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
has a stabilizing effect. Backward curved blades may be used for the same reason
as for type e.
d. Turbine with blades are inclined 45" (usually). Constructions with two to eight
blades are used, six being most common. Combined axial and radial flow are
achieved. Especially effective for heat exchange with vessel walls or internal
coils.
e. Curved blade turbines effectively disperse fibrous materials without fouling.
The swept back blades have a lower starting torque than straight ones, which is
important when starting up settled slurries. f. Shrouded turbines consisting of a
rotor and a stator ensure a high degree of radial flow and shearing action, and are
well adapted to emulsification and dispersion. g. Flat plate impellers with
sawtooth edges are suited to emulsification and dispersion. Since the shearing
action is localized, baffles are not required. Propellers and turbines also are
sometimes
provided with sawtooth edges to improve shear. b. Cage beaters impart a cutting
and beating action. Usually they are mounted on the same shaft with a standard
propeller. More violent action may be obtained with spined blades.
SHAFT
The shaft is vital component of the agitator and frequently limits its mechanical
performance. In addition to transmitting torque, the shaft undergoes bending, and
if not stiff enough or rigidly supported, it may vibrate badly and cause discomfort
to personnel or damage to the equipment. Therefore it must be analyzed for
combined torsional and bending stresses, deflection, and critical speed and must
be selected to meet the limiting criteria for each. Inadequately in these respects
can result in failure of the shaft by overstress, failure of the seal due to excessive
shaft bending, or failure of bearings due to wear or impact Torsional and bending
stresses calculation
COUPLINGS
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Most agitators with long overhung shaft have rigid couplings to
connect the agitator shaft and the gear reducer output shaft. The coupling facilities
shipment, installation, removal and servicing of the agitators. Although it is an
innocuous appearance block of metal, care in its design and fabrication can
contribute significantly towards the satisfactory performance of the agitator. The
reasons for this become manifest from the requirement that the rigid coupling
must meet
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
1- the coupling must be capable of transmitting the agitator torque
2- the coupling must provide at least as much rigidity as the shaft if the
critical speed of the agitator is not to be reduced .
3- the coupling must strong enough to withstand the bending moments
imposed upon it by the unbalanced hydraulic and centrifugal force.
4- The coupling must provide good alignment between the shafts being
connected
5- Because it must be assembled and disassembled in the field, frequently
under inconvenient working condition, the coupling should be relatively
easy to take apart and reassembled, and should preferably be self-aligning.
6- The coupling must be capable of taking the thrust due to weight of the
agitator.
DRAFT TUBES
A draft tube is a cylindrical housing around and slightly larger
in diameter than the impeller. Its height may be little more than the diameter of
the impeller or it may extend the full depth of the liquid, depending on the flow
pattern that is required. Usually draft tubes are used with axial impellers to direct
suction and discharge
Streams. An impeller-draft tube system behaves as an axial flow pump of
somewhat low efficiency. Its top to bottom circulation behavior is of particular
value in deep tanks for suspension of solids.
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
calculations
Sodium hydroxide concentration calculation
NaOH added = 10.656 Kgmol = 426.25 Kg
Water added = 66.48 Kgmol = 1196.65 Kg
Density of NaOH =
1023.7 Kg/M3
Density of water =
997Kg/M3
Total volume of the mixture = V1+V2+dV
whereV1 is partial volume of NaOH
V2 is partial volume of water
dV is change in volume due to solubility
V
=
426.25
---------- +
1023.7
1196.65
----------997
= 1.617 M3
Reactor volume and dimensions calculations
=
=
CA0
10.656
----------1.617
6.59 Kgmol/M3
= 6.59 gmol/lit
V
Xa
-------- = --------- = 342
Fa0
- Ra
V = 0.12233*25*342
= 1.055 M3
(from graph)
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
V =
2.1
(50% reactor is filled)
prove it
:
Basic equation used for calculating reactor volume =
XA
(------------)
- rA
Vf = FA0
Vf = 1.35 m3
Vf = volume of fluid
V = 2.1 m3 (73% fill)
V = volume of vessel
D = 1.2 m
D = diameter of
vessel
H = 1.2 m
H = height of fluid
L= 1.87 m
L = length of vessel
Selection and geometry of Impeller
I selected the 300 curved, square pitched turbine blade with the
Following specifications:
S-1 =
Da/Dt
S-2 =
E/Dt
S-3 =
L/Da
S-4 =
W/Da
S-5 =
J/Dt
S-6 =
H/Dt
No. of baffles = 4
= 0.33
= 0.33
= 0.25
= 0.20
= 0.0833
= 1.00
(no clearance, not twisted but adjacent with
wall of the vessel )
Power calculation:
P =Np0(Nr3)(Da)5 = Kt (Nr3)(Da)5
= 0.34 Kw =0.45 hp
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
= 0.55 (82 % efficiency of the motor)
SHELL THICKNESS
X
=
Pri
Cc
(SE j 0.6 P)
P
=
Pressure of system
ri
=
Radius of shell
S
=
Stress of material
Ej
=
Efficiency of joint
Cs
=
Corrosion rateik
=
101.3 7.7 1.292
0.002
160000 0.85 0.6 7.7 101.3
=
5.502 mm
X
HEAD THICKNESS
Pressure at the lease
=
gh
=
1080.46 × 9.8 × 4.43
=
46.907 KPa
Total pressure at base
=
827.697 KPa
t
=
Pi D i
2 S E j 0.2 P
827.69 1.92
2 160000 0.85 0.2 827.69
=
=
5.84 mm
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Power number, N, = Pg,/N3D5p, against Reynolds number, NRe = NDzp/p, for
several kinds of impellers: (a) helical shape (Oldshue, 1983); (b) anchor shape
(Oldshue, 1983); (c) several shapes: (1) propeller, pitch equalling diameter,
without
baffles; (2) propeller, s = d, four baffles; (3) propeller, s = 2d, without baffles; (4)
propeller, s =2d, four baffles; (5) turbine impeller, six straight blades, without
baffles; (6) turbine impeller, six blades, four baffles; (7) turbine impeller, six
curved blades, four baffles; (8) arrowhead turbine, four baffles; (9) turbine
impeller,inclined curved blades, four baffles; (10) two-blade paddle, four baffles;
(11) turbine impeller, six blades, four baffles; (12) turbine impeller with stator
ring; (13) paddle without baffles (data of Miller and Mann); (14) paddle without
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
baffles (data of White and Summerford). All baffles are of width 0.1D [after
Rushton, Costich,
and Everett, Chem. Eng. Prog. 46(9), 467 (1950)l.
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Specification Sheet
Identification:
Item :
Item no:
No.required:
CFSTR ( Continuous Flow Stirred Tank Reactor)
R-01
01
Function:
Formation of sodium phenate from carbolic acid and caustic soda
Operation:
Continuous
Type:
Agitator cylindrical vessel
Design Data:
Vessel:
Working Volume
Design Volume
Temperature (Process temperature)
Design Temperature
Working Pressure
Design Pressure
Dia of Vessel
Height of vessel
Working height
Height to dia ratio
Type of head
Thickness of cylindrical protion
Thickness of bottom head
No. of baffle
Width of Baffle
Height of baffle
1.35 m3
1.86 m3
99˚C
109˚C
1 atm
1.5 atm
1.2 m
1.56 m
1.2 m
1.3
Torispherical
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Agitator:
Following specifications:
S-1 =
Da/Dt
S-2 =
E/Dt
S-3 =
L/Da
S-4 =
W/Da
S-5 =
J/Dt
S-6 =
H/Dt
No. of baffles = 4
= 0.33
= 0.33
= 0.25
= 0.20
= 0.0833
= 1.00
(no clearance, not twisted but adjacent
with wall of the vessel )
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Flash tank design
The name originate from the fact that a liquid at a pressure equal to or greater than
its bubble point pressure flashes or vaporizes, when the pressure is reduced below
its bubble point pressure producing two phase system of liquid and vapor in
equilibrium.
Basic equations used in flash calculation are
L+ V =
Ki =Yi/Xi
1 (material balance equation)
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Where Ki equilibrium constant
Yi vapor phase composition
Xi liquid phase composition
From Raults and Henry Law
YiiP = XiγiPisat
I = 1 for low to moderate pressure
γi = 1 assumption as no data is available in
literature
Zi Ki
Yi =
---------------------1+V(Ki – 1)
Zi
Xi =
----------------------1+V (Ki – 1)
for flash calculation necessary condition
P dew < P< Pbubble
Very important equation used in flash calculation
i - idew
γi - γidew
---------------γi bubble -γidew
=
---------------I bubble - idew
V-1
P – P dew
=
--------------------
Pbubble - Pdew
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
=
--------------0
- 1
P – P dew
=
----------Pbubble - Pdew
P -
P dew
V = --------------------P bubble – P dew
P bubble
=
X1P1sat +X2P2sat ……..
1
P
=
dew
-----------------Y1/P1sat+Y2/Psat …
Throttling Valves
Globe Valve is an economical throttling
valve. Its heavy duty design provides for
long service life. The in-line globe design
causes
relatively
high
pressure
drops,
however this is a desirable valve due to its
economy and reliability.
Features
1- Slow closing
2- Prevents water hammer in PVC piping
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
3- Heavy Duty Construction
4- Long service life
5-efficient throttling with minimum wire drawing or disk or seat erosion
6-available in multiports- short disk travel and fewer turn to operate, saving
time and
wear on stem and bonnet
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Jet Ejector
The two most common ejectors are operated by water or steam. The liquid
ejectors are used for creating a modest vacuum or for mixing liquids. The steam
ejectors is important in creating and holding a vacuum in a system. Ejectors have
no moving parts and operated by the action of one high pressure steam entraining
and other vapors (or liquids) at low pressure into a moving stream and thereby a
removing them from the process system at an intermediate pressure
Feature
Ejectors have the following features which make them good choice
for continuously producing economical vacuum condition.
1- They handle wet, dry or corrosive vapor mixtures
2- They develop any reasonable vacuum needed for industrial operation.
3- All sizes are available to match any small or large capacity requirement.
4- Their efficiencies are reasonable to good.
5- Have no moving parts, hence maintenance low, operation fairly constants
when corrosion is not a factor.
6- Quiet operation.
7- Stable operation within design range
8- Installation cost relatively low when compare to mechanical vacuum
pumps.
9- Space requirement is small
10- Simple operation.
Types
Ejectors may be single or multi-stage. The extra stages, with or
without inter stage condensing of steam; allow the system to operate at lower
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
absolute pressure than a single stage unit. Various combinations of series of jets
with no inter-condensing can be connected to jets with inter-condensers or after
condensers to obtain various types of the operation and steam economy.
Material of construction
Since the ejector is basically simple in construction, it is available
in many material suitable for handling corrosive vapors. Standard materials
include cast iron, meehanite, cast steel, and bronze for the body and diffusers
depending upon the pressure and temperature rating. The nozzle is usually
stainless steel or monel. Other material of construction include porcelain, carbon
graphite, impregnated graphite, synthetic resins, glass and special metal of all
types
DEMISTERS OR IMPINGENT SEPARATOR
As the descriptive name suggests, the impingement separator
allows the particles to be removed to strike some type of surface. This action is
better accomplished in pressure system where pressure drop can be taken as a
result of turbulence which necessarily accompanies the removal action. Particles
removed in stream line flow is less efficient than for turbulent flow and not be
effective if the path of travel is not properly well baffled.
There is basically three construction type for impingement separators.
1-wire mesh
2- Plates (curved, flat or special shaped)
3-packed impingement beds
A demister pad resembles a giant brillo pad with the soap. Many process plants
have discarded demister pads lying around their scrape heaps. The theory of
operation of demister is simple. Vapors and droplets of liquids strike the demister
pad with a sustainable velocity. The force of this impingement velocity causes the
tiny droplets. The heavier droplets of liquid to coalesce into large droplets.
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
For knockout drum with a demister pad it apparently must have K-value of at
least
0.15 to 0.20 When a demister plugs it increases the pressure drop of the vapor, but
pressure drop cannot increase a lot because the demister will break. Demister
failure creates two problems.
1- The dislodged section of the demister pad are blown into down stream
equipment as into suction of the centrifugal wet gas compressor
2-the failed demister promotes high localized velocities. Vapors blows through the
open areas of the vessel. The remaining section of the demister pad impedes vapor
flow. The resulting high localized velocities of vapors creates more entrainment
than we could have without any demister
Steam trap
A steam trap is self actuating automatic drain valve in a steam distribution system
that performs the following functions.
1- Removal of condensate
2-Remove air or other noncondensible gases
3- Prevents or limits the loss of steam
Condensate (water) is formed when steam condenses to release latent heat. Some
heat is released in the distribution system in the form of unavoidable losses. Most
of the heat is utilized in the process equipment. Once steam has condensed, the
hot condensate must be removed immediately as it hinders effective heat transfer
from the incoming steam. By selectively purging a steam system of its condensate
and noncondensible, steam trap helps maintain high heat transfer coefficient in
equipment without losing live steam
In the recent years user interest in steam trap has closely paralleled
the increase in energy cost. High fuel cost associated with the malfunctioning trap
cannot be ignored. Spiraling cost have highlighted the need for optimum steam
trap selection and application. Experience indicates that improper selection and
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
application are the most frequent cause of the trap failure and steam loss. The
installation or the replacement of steam trap has a pay back period of as little as
three to six months.
Classification of steam trap
There are three basic type of steam traps using different physical
principle to distinguish between steam and condensate.
Thermostatic steam trap
These traps are actuated by the temperature sensitive
which operate on the basis that the steam is hotter than condensate, air, and other
noncondesibles. There are three types of thermostatic traps.
1-
Liquid expansion
2-
Balanced pressure
3-
Bimetallic
Mechanical steam trap
These traps operate on the difference of density between
steam and condensate. The two important type of mechanical steam traps are
1- ball float
2- bucket
Thermodynamic steam trap
These traps are operates on the facts that flash steam is
produced when pressure is reduced on the hot condensate. The release of flash
steam causes the discharge to close. There are the following further types.
1- Disk type
2-
Piston type
3-
Lever type
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
FLASH CALCULATION
data
temperature
v.p.of waterKPa
v.p.of
phenol
Kpa
v.p.of
100
101.33
fluid composision
name
composition
water
0.87
3.51
phenol
Na-
0.0137
PheKPa
2.07
Phe
0.1182
Pbubble Kpa
Pdew Kpa
88.45
14.36
Na-
vapor
0.856391
liquid
0.143609
composition
0.975529
0.00729
0.0139688
1.028442
comp.
0.0220693
0.051924
0.871002
0.945
KPRESURE Kpa
25
component
water
phenol
Na-Phe
values
4.053
0.1404
0.0828
Yi
0.9786
0.007089
0.014361
1
Total mole for 25-ton per day = 88.85656*25 = 2221.4 Kgmol
Total moles in tank
=
Total weight
= 318.995(0.9*116+0.061*94+0.02818)
=
318.995 Kgmol
35293.41 Kg
Weight required for 1.5-batch of autoclave = 35293.41/4 = 8823.35 Kg
Average density = 0.90*1258+0.061*1023+0.028*994
=
1222.25 Kg/m3
Xi
0.028531
0.061549
0.9099
1
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Filled volume
= 7.2174 m3
% fill
total volume
= 45 %
= 16 m3
L = 5m , D = 2m
Some more specifications
Height of liquid
=
Height of inlet pipe
=
Height of demister pad =
Thickness of demister pad =
Material of demister pod
=
Height of outlet
=
Steam pressure
=
DESIGN OF DISTILLATION COLUMN
The detailed process design of the carbolic acid column is given below. The
pictorial Representation of the column is given in fig. The feed to the column is a
mixture of carbolic acid and sodium phenolate. The compositions of the
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
components are given below. The top and bottom product, both are the required
products used in reactor one and in autoclave (second reactor).
I. Thermodynamics: The primary requirement while designing mass transfer
contact equipment is the
Thermodynamic equilibrium data. The data required is in the Vapor-Liquid
Equilibrium
(VLE) data for the carbolic acid and sodium phenolate system. The X-Y curve is
shown in the fig. To
Develop the VLE data, a model was used.
yi pt =
i xi Pi sat --------------------------(1)
Where,
yi = mole fraction of component “i” in vapor.
pt = total system pressure.
i = activity coefficient of component “i” in
liquid.
xi = mole fraction of component “i” in liquid.
Pi sat = saturation vapor pressure of component “i”.
The equilibrium vapor pressure was evaluated using correlations given in
literature. The correlation was based on the critical properties of the components.
The two components carbolic acid and sodium phenolate form a highly non-ideal
system. To accommodate this nonideality, an activity coefficient term was used
for the liquid phase. The activity coefficient was evaluated using the UNIFAC
model. Since the evaluation of the VLEdata is highly iterative, an algorithm was
developed which was solved using a computer program. The gas phase was
assumed to be ideal. This is a valid assumption since the column is at 1
atmosphere pressure (760 mm Hg. abs.). The boiling points of the two
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
components require the column to be operated at 1 atmosphere. The operating
pressure was chosen to be 760 mm Hg (abs).
The following questions must be answered before going into detailed design of
the distillation column
1- what column is selected packed or tray column ?
Answer
Tray column would be better choice in situation facing due to the following
reasons
1- stage efficiency can be determined experimentally in packed column
because
ŋ =f(type and sizing of packing, fluid rates, fluid properties, column diameter,
operating temperature and pressure, extent of liquid dispersion……)
Where as a number of correlations are available for tray
column efficiency discussed by the famous authors. Coulson and Richardson,
Ludwig, Perry, Peter and Timmerhaus, conceptual design of distillation
column and more…..
2-tray column can be designed over a wide range without flooding
3-total dry weight of the tray column is less than the packed column
4-design information for tray column id readily available as compared to
packed column. This made path of working easy for me when I decided tray
column
5-if chances of foam formation are more then packed column would be given
preference, but in situation facing me no foam formation (surface tension at
different temperature and pressure would dictate foam formation criteria)
6- Thermal expansion chances can be easily handled with tray column instead
of packed column.
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
7- Equilibrium Data for large compounds as well as my compound are
available in literature and this data is more reliable for tray column instead of
packed column
What type of tray is selected sieve tray, bubble cap, or valve tray ?
Answer
I decided sieve tray because of the following reasons
1- Manufacturing cost is low
2-operating cost is low
3-simple to construct
4-minmum entrainment as compare to bubble cap and valve tray
5-less pressure drop as compare to other
6-most commonly used
Glossary of notations used:
F = molar flow rate of Feed, gm
D = molar flow rate of Distillate, gmol/hr.
W = molar flow rate of Residue, gmol/hr.
xF = mole fraction of phenol in liquid/Feed.
yD = mole fraction of phenol in Distillate.
xW = mole fraction of phenol in Residue.
MF = Average Molecular weight of Feed, g/gmol
MD = Average Molecular weight of Distillate, g/gmol
MW = Average Molecular weight of Residue, g/mol
RRm = Minimum Reflux ratio
RR = Actual Reflux ratio
LR = Molar flow rate of Liquid in the Enriching Section, gmol/hr.
VR = Molar flow rate of Vapor in the Enriching Section, gmol/hr.
LS = Molar flow rate of Liquid in Stripping Section, gmol/hr.
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Vs = Molar flow rate of Vapor in Stripping Section, gmol/hr.
q = Thermal condition of Feed
ρl = Density of Liquid, kg/m3.
ρg = Density of Vapor, kg/m3.
ql = Volumetric flow rate of Liquid, m3/s
qv = Volumetric flow rate of Vapor, m3/s
l = Viscosity of Liquid, cP.
Tl = Temperature of Liquid, 0K.
Tv = Temperature of Vapor, 0K.
II. Preliminary calculations:
Feed = 9915 gmol/h
xF = 0.67, MF = 101.16g/gmol.
D = 6783.89gmol/hr, xD = 0.97, MD = 94.66g/gmol.
W=3131.1gmol/hr, xW = 0.02, MW =115.56g/mol.
Basis: 1 Hour Operation.
From the graph
Rm = 1.5
Let, R= 2.1 *Rm
estimated )
RR= 1.5×2.0= 3.2
Number of Ideal trays = 17
Number of Ideal trays in Enriching Section = 8
Number of Ideal trays in Stripping Section = 9
Now, we know that,
RR = R/ D
=> R =RR×D
i.e., R= 3.2*6783.89 = 21705gmol/h
L= Liquid flow rate on the Top tray = 21705gmol/h
VR= L + D
(from trial this value is
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
= (R+1)*D = 4.2*6783.89mol/hr = 28488.6gmol/h
VR = Gas flow rate in the Enriching Section = 28488.6gmol/h
Since feed is Liquid, entering at bubble point,
q= (HV-HF) / (HV-HL) = 1
Now,
Slope of q-line = q/ (q-1) = 1/ (1-1) = 1/0 = .
Now we know that,
LS = F + L R
LS = 9915+21705.6=31619.6 gmol/h
Therefore, liquid flow rate in the Stripping Section = 31619.6 gmol/h
Also, we know that,
VS = [(q-1) ×F] + VR
VS = [(1-1) ×F] + VR
VS = VR = = 28488.6gmol/h
Therefore, the flow rate of Vapor in the Stripping Section = = 28488.6gmol/h
IV. Design Specification:
Tray Hydraulics
The design of a sieve plate tower is described below. The equations and
correlations are borrowed from the 6th and 7th editions of Perry’s Chemical
Engineers’ Handbook, plant design and economics by Peter and Timmerhaurse,
ludwick, unit operation of chemical engineering by McCabe and Smith, Coulson
and Richardson’s book, separation techniques by C.G. King The procedure for
the evaluation of the tray parameters is iterative in nature. Several iterations were
performed to optimize the design.
The final iteration is presented here.
1. Tray Spacing, (ts):
Let ts = 457mm (18in).
2. Hole Diameter, (dh):
Let dh = 5 mm.
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
3. Hole Pitch (lp):
Let lp = 3× dh
i.e., lp = 3×5 = 15 mm.
4. Tray thickness (tT):
Let tT = 0.6× dh
i.e., tT = 0.6×5 = 3 mm.
5. Ratio of hole area to perforated area (Ah/Ap):
Refer fig 6.3Now, for a triangular pitch, we know that
Ratio of hole area to perforated area (Ah/Ap) = ½ (/4×dh2)/ [(√3/4) ×lp2]
i.e., (Ah/Ap) = 0.90× (dh/lp)2
i.e., (Ah/Ap) = 0.90× (5/15)2
i.e., (Ah/Ap) = 0.1
Thus,
(Ah/Ap) = 0.1
6. Plate Diameter (Dc):
The plate diameter is calculated based on the flooding considerations
L/G { g/ l}0.5 = 0.11
Now for,
L/G { g/ l}0.5 = 0.11 and for a tray spacing of 457 mm.
We have from the flooding curve, ---------- (fig.18.10, page 18.7, 6th edition
Perry.)
(Plant design and economics by Peter and
Timmerhaus)
Flooding parameter,
Now,
Csb, flood = 0.07 m/s.
Unf = Csb, flood × (σ/ 20) .0.2 [( l - g) / g] 0.5---- {eqn. 18.2, page
18.6,
6th edition
Perry.}
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
(Plant design and economics by Peter and
Timmerhaus)
Where,
Unf = gas velocity through the net area at flood, m/s (ft/s)
Csb, flood = capacity parameter, m/s (ft/s, as in fig.18.10)
σ= liquid surface tension, mN/m (dyne/cm.)
l = liquid density, kg/m3 (lb/ft3)
g = gas density, kg/m3 (lb/ft3)
Now, we have,
σ= 28.57 dyne/cm.
ρl = 1023 kg/m3.
ρg = 2.52 kg/m3.
Therefore,
Unf = 0.07× (28.57/20)0.02 *[987-2.52)/ 2.52]0.5
i.e., Unf = 4.87 ft/s = 1.49 m/s.
Let
Actual velocity, Un= 0.8×Unf
i.e., Un = 1.19 m/s
Now, volumetric flow rate of Vapor
qo = 28488.6*94.5 / (3600×2.52*1000) = 0.3 m3/s.
Net area available for gas flow (An)
Net area = (Column cross sectional area) - (Down comer
area.)
An = Ac - Ad
Thus,
Net Active area, An = qo/ Un = 0.3/1.119 = 0.252 m2.
Let Lw / Dc = 0.75
Where, Lw = weir length, m
Dc = Column diameter, m
,
θc = 2×sin-1(Lw / Dc) = 2×sin-1 (0.75) = 97.180
Ac =(3.14/4) × Dc2= 0.7854×Dc2 , m2
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
And,
θc = angle
Ad = [(/4) × Dc2 × (θc /3600)] - [(Lw/2) × (Dc/2) ×cos (θc /2)]
Ad = [0.7854× Dc2 × (97.180/3600)]-[(1/4) × (Lw / Dc) × Dc2 × cos
(97.180)]
Ad = (0.2196× Dc2) - (0.1241× Dc2)
Ad = 0.0955×Dc2, m2
Since,
An = Ac -Ad
0.252 = (0.7854×Dc2) - (0.0955× Dc2)
i .e., 0.6895× Dc2 = 0.252
Dc2 = 0.252/ 0.6895 = 0.365
Dc = 0.6 m
Take Dc = 0.635 m
Since Lw / Dc = 0.75,
Lw = 0.75× Dc = 0.75×0.635 = 0.476 m.
Therefore, Lw = 0.476 m.
Ac = 0.7854×0.6352 = 0.316 m2
Now,
Ad = 0.0879×0.635 = 0.0354 m2
An = Ac - Ad
An =0.316-0.0354
An = .2806 m2
7. Perforated plate area (Ap):
Active area (Aa)
Aa = Ac - (2×Ad)
Aa = 0.316- (2×0.0354)
Aa = 0.245 m2
Lw / Dc = 0.476/ 0.635 = 0.75
θc = 97.18 0.
θc = angle
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
θ1=180 0 - 97.18 0 = 82.82 0
θ1 =
angle
Acz = 2× Lw× (thickness of distribution)
= 5 – 20% of Ac
Where Acz = area of calming
zone, m2
Acz = taking 10% of Ac = 0.02833
Also,
Awz = {(Л/4)Dc2 (ά/3600)}-{Л/4× (Dc-0.05)2× ά/3600}
Where Awz = area of waste periphery, m2
i.e., Awz = 2 – 5 % of Ac, taking 2% of Ac,
i.e., Awz = 0.0.0057 m2
Now,
Ap = Ac - (2×Ad) - Acz – Awz
Ap = 0.2833 – 2*0.0354 - 0.02833 - 0.0.0057 =0.1784 m2
Thus, Ap = 1.720 m2
8. Total Hole Area (Ah):
Since,
Ah / Ap = 0.1
Ah = 0.1× Ap
Ah = 0.1× 0.1784 =0.01784m2
Thus, Total Hole Area = 0.01784 m2
Now we know that,
4*Ah = nh × (.Лdh2)
Where nh = number of
holes.
nh = (4×0.01784)/ (.3.14×0.0052)
⇒nh = 909
Therefore, Number of holes = 909.
9. Weir Height (hw):
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Let hw = 45 mm.
10. Weeping Check
All the pressure drops calculated in this section are represented as mm head of
liquid on the plate. This serves as a common basis for evaluating the pressure
drops.
Notations used and their units:
hd = Pressure drop through the dry plate, mm of liquid on the plate
uh = Vapor velocity based on the hole area, m/s
how = Height of liquid over weir, mm of liquid on the plate
hσ= Pressure drop due to bubble formation, mm of liquid
hds= Dynamic seal of liquid, mm of liquid
hl = Pressure drop due to foaming, mm of liquid
hf = Pressure drop due to foaming,
Df = Average flow length of the liquid, m
Rh = Hydraulic radius of liquid flow, m
uf = Velocity of foam, m/s
(NRe) = Reynolds number of flow
f = Friction factor
hhg = Hydraulic gradient, mm of liquid
hda = Loss under down comer apron, mm of liquid
Ada = Area under the down comer apron, m2
c = Down comer clearance, m
hdc = Down comer backup, mm of liquid
Calculations:
Head loss through dry hole
hd = head loss across the dry hole
hd = k1 + [k2× (ρ g/ρl) ×Uh2] --------- (eqn. 18.6, page 18.9, 6th
edition Perry)
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
where Uh =gas velocity through
hole area
k1, k2 are constants
For sieve plates
k1 = 0 and
k2 = 50.8 / (Cv)2
where Cv = discharge coefficient, taken from fig. 18.14, page 18.9, 6th edition
Perry).
Now,
(Ah/Aa) = 0.01784/ 0.245 m2 = 0.073
also tT/dh = 3/5 = 0.60
Thus for (Ah/Aa) = 0.07993 and tT/dh = 0.60
We have from fig. edition 18.14, page 18.9
6th Perry.
Cv = 0.730
k2 = 50.8 / 0.7302 = 95.3275
Volumetric flow rate of Vapor at the top of the Enriching Section
qt = 0.3 m3/s.
Velocity through the hole area (Uh):
Velocity through the hole area at the top = Uh, = qt /Ah
= 0.3/0.01784 = 16.8 m/s
Velocity through hole area should be minimum because at low gas flow rate
weeping is
observed.
Now,
hd, = k2 [ρg/ρl] (Uh )2
= 95.3275(2.91/1011) 16.82
hd, top = 85.24 mm clear liquid. -------(minimum at top)
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Head Loss Due to Bubble Formation
hσ = 409 [σ/ (ρldh)] --- (eqn. 18.2.a, page 18.7, 6th
edition Perry)
where σsurface tension, mN/m
(dyne/cm)
dh =Hole diameter, mm
ρl = average density of
liquid in the
section,
kg/m3
= (987+1141)/2
= 1064 kg/m3
hσ=409 [17.4565 /(777.92 x5)]
hσ= 2.714 mm clear liquid
Height of Liquid Crest over Weir:
how = 664_)w [(q/Lw)2/3]-----------( eqn. 18.12.a, page 18.10,
6thedition Perry
q
= liquid flow rate at top, m3/s
= 17747.8/
(3600788.86)
= 6.24910-3 m3/s
q = 99.05 gal/min(or
GPM).
Lw = weir length = 1.425 m = 4.675 ft
q’/Lw2.5 = 99.05/ (4.675) 2.5 = 2.096
now for q’/Lw2.5 = 2.096 and Lw /Dc =0.75
we have from
fig.18.16,
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
page 18.11, 6th
edition Perry
Fw= correction factor =1.025
how = 1.025664[(6.24910-3)/1.425]2/3
how = 18.23 mm clear liquid.
Now,
(hd + hσ) = 85.24 + 2.714 = 87.95 mm ------ Design value
(hw + how) = 45 + 18.23 = 63.23 mm
Also, Ah/Aa = 0.08 and (hw + how) = 63.23 mm
The minimum value(hd + hσ) of required is calculated from a graph given in
Perry,
plotted against Ah/Aa. we have from fig. 18.11, page 18.7, 6th edition Perry
(hd + hσ) min = 16.0 mm ------- Theoretical value.
The minimum value as found is 16.0 mm.
Since the design value is greater
than
the minimum value , there is
no
problem ofweeping.
Down comer Flooding:
hds =hw + how + (hhg /2) ------- (eqn 18.10, page 18.10, 6th edition Perry)
Where,hw = weir height, mm
hds = static slot seal (weir height minus
height
of top of slot above plate floor,
height
equivalent clear liquid, mm)
how = height of crest over weir, equivalent
clear
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
liquid, mm
hhg = hydraulic gradient across the plate,
height of
equivalent clear liquid, mm.
In the above equation how is calculated at bottom of the section and since the
tower is
operating at atmospheric pressure, hhg is very small for sieve plate and hence
neglected.
Calculation of how at bottom conditions of the section:
q = liquid rate at the bottom of the section, m3/s
= 31619*116/(3600*1258*1000)=8.1x10-4 m3/s
= (8.1x10-4)/ (6.309*10-3) = 7.7 gal/min
Lw = weir length = Lw = 0.476 m.= 1.56 ft.
q’/Lw2.5 =7.7/ (1.56)2.5 = 3.14
now for q’/Lw2.5 = 3.14and Lw /Dc =0.75
we have from fig.18.16, page 18.11, 6th edition
Perry
Fw= correction factor =1.030
Thus, how = 1.03664[(8.1x10-4)/0.47]2.3
how = 19.33 mm clear liquid. ----- (maximum at the bottom of section).
Therefore, hds = 45 +19.33 = 64.33 mm.
Now, Fga = Ua ρg0.5
Where Fga = gas-phase
kinetic energy factor,
Ua = superficial gas velocity, m/s
(ft/s),
ρg = gas density, kg/m3 (lb/ft3)
Here Ua is calculated at the bottom of the
section.
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Ua = (Gb/ρg)/ Aa = (28072/2.91) /(0.245x3600) =
1.24 m/s
Thus, Ua = 4.06 ft/s
ρg = 2.91 kg/m3 = 2.91/ (1.60184610-1) = 0.181
lb/ft3
Fga = 4.06(0.181)0.5
Fga = 1.72
Now for Fga = 1.72, we have from fig. 18.15, page 18.10 6th edition Perry)
Aeration factor = = 0.6
Relative Froth Density = t = 0.20
Now hl’= hds ---- (eqn. 18.8, page 18.10, 6th edition Perry)
Where, hl’= pressure drop through the aerated mass over and around the
disperser,
mm liquid,
hl’= 0.664.33 = 38.598 mm.
Now,
hf = hl/t ------- (eqn. 18.9, page 18.10, 6th edition Perry)
hf = 38.598/ 0.20 = 192.99 mm.
Head loss over down comer apron:
hda = 165.2 {q/ Ada}2 ----- (eqn. 18.19, page 18.10, 6th edition Perry)
Where, hda = head loss under the down comer apron, as millimeters of liquid,
q = liquid flow rate calculated at the bottom of section, m3/s
and Ada = minimum area of flow under the down comer apron, m2
Now,
q = 18950.03/(3600776.98) = 6.77410-3 m3/s
Take clearance, C = 1/2” = 12.5 mm
hap = hds - C = 64.33 – 12.5 =51.83 mm
Ada = Lw x hap = 0.4710-3 = 24.36*10-3 m hda2
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
hda = 165.2[6.77410-3 / 24.36x 10-3] 2
= 12.75 mm
ht = total pressure drop across the plate (mm liquid)
= hd + hl`
= 85.24 + 38.598
= 123.838 mm
Down comer backup:
hdc = ht+ hw + how + hda + hhg ---- (eqn 18.3, page 18.7, 6th edition Perry)
Where, hdc = height in down comer, mm liquid,
hw = height of weir at the plate outlet, mm liquid,
ho =height of crest over the weir, mm liquid,
hda = head loss due to liquid flow under the down comer apron, mm liquid,
hhg = liquid gradient across the plate, mm liquid.
hdc =123.83 + 45 + 19.33 + 12.75 + 0
= 201 mm
Let dc = average relative froth density (ratio of froth density to liquid density)=
0.5
hdc = hdc /
= 201/ 0.5 = 402 mm which is less than the tray spacing of 457 mm.
Hence no flooding in the enriching section
V. EFFICENCIES: (AIChE Method)
A) Enriching Section:
Point Efficiency, (Eog):
Eog = 1-exp (-Nog) ----- (eqn. 18.33, page 18.15, 6th edition Perry)
Where Nog = Overall transfer units
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Nog = 1/ [(1/Ng) +(λ/Nl)] ---- (eqn. 18.34, page 18.15, 6th edition Perry)
Where Nl = Liquid phase transfer units,
Ng = Gas phase transfer units
λ =(m×Gm)/ Lm = Stripping factor,
m = slope of Equilibrium Curve,
Gm = Gas flow rate, mol/s
Lm = Liquid flow rate, mol/s
Ng= (0.776 + (0.00457×hw) – (0.238×Ua * ρg0.5)+ (104.6×W))/ (NSc, g)0.5 ----(eqn. 18.36,
page 18.15, 6th edition
Perry)--- *
hw = weir height = 45.00 mm
Ua = Gas velocity through active
area, m/s
Ua= (Avg. vapor flow rate in kg/hr)/ (3600×Avg. vapor density ×active area)
= (28488.6*116/1000)/ (3600×0.245×2.925)
Ua = 1.28 m/s
Df = (Lw + Dc)/2 = (0.635+0.47)/2 = 0.55 m
Average Liquid rate = 2040.27 kg/hr
Average Liquid Density =991 kg/m3
q = 2040.36/ (3600x991) =5.71 x 10-0.4 m3/s
W = Liquid flow rate, m3/ (s.m) of width of flow path on the plate,
= q/Df = 5.71*10-4/0.55 = 1.04×10-3 m3/ (s.m)
NSc g = Schmidt number =µg (ρg×Dg) = 0.66
Number of gas phase transfer units
Ng= (0.776 + (0.00457×45) – (0.238×1.28×2.925 0.5) + (104.6×1.04×10-3))/
(0.66)0.5
Ng = 0.744
Number of liquid phase transfer units
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Nl = kl× a×θl ----- (eqn 18.36a, page 18.15, 6th edition Perry)
Where kl = Liquid phase transfer coefficient kmol/ (sm2 kmol/m3) or m/s
a = effective interfacial area for mass transfer m2/m3 froth or spray on the plate,
θ1= residence time of liquid in the froth or spray, s
θ l = (hl×Aa)/(1000×q) ---- (eqn. 18.38, page 18.16, 6th edition Perry)
q = liquid flow rate, m3/s = 5.71*10-4 m3/s
hl = hl’ = 38.08 mm
Aa = 0.47 m2
θl = (38.08×0.47 )/(1000×5.71*10-4) =31.34 sec
kl ×a = (3.875×108×DL)0.5× ((0.40×Ua×ρg0.5) + 0.17)--- (eqn. 18.40a, page
18.16,
6th edition Perry)
DL= liquid phase diffusion coefficient, m2/s
kl ×a = (3.875×108×8.08×10-9)0.5× ((0.40×1.28×2.9250.5) + 0.17)
kl ×a = 1.832 m/s
Nl = kl× a×θl
i.e., Nl = 1.833×31.34 =57.13
Slope of equilibrium Curve
mtop = 0.4375
mbottom = 0.84
Gm/Lm = 1.2
λt = mt × Gm/Lm =0.4375*1.2=0.525
λb = mb×Gm/Lm = 0.84*1.2 = 1.002
λ= 0.76
Nog = 1/[(1/Ng_+λ/Nl)]
= 1/[(1/0.774) + (0.76/57.16)]
Nog = 1.6
Eog = 1-e-Nog = 1-exp (-Nog)
= 1-e-1.037 = 1-exp (-1.037)
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Eog = 0.67
∴Point Efficiency = Eog = 0.67
2. Murphree Plate Efficiency (Emv):
Now,
Pelect number =NPe = Zl2/ (DE×θ l)
Where Zl = length of liquid travel, m
DE = (6.675 x 10-3× (Ua)1.44) + (0.922 × 10 -4× hl) - 0.00562----- (eqn. 18.45,
page 18.17,
6th
edition Perry)
where DE = Eddy diffusion coefficient, m2/s
DE = (6.675 x 10 -3× (1.266)1.44) + (0.922 × 10 -4× 38.08) - 0.00562
DE = 7.265×10-3 m2/s
Also Zl = Dc× cos (θc/2)
= 0.635× cos (97.18 0/2)
= 0.46 m
NPe = Zl2/ (DE× θl)
= 0.462/ (7.265×10-3 × 57.13)
NPe = 5.1
λ×Eog = 0.76 x 0.67 = 0.502
now for λ×Eog = 0.60 and NPe = 5.1
We have from fig.18.29a, page 18.18, 6th edition Perry
Also available in seventh edition
Emv/ Eog = 1.28
Emv = 1.28× Eog = 1.28×0.67 =0.81
Murphree Plate Efficiency = Emv = 0.81
3. Overall Efficiency ( EOC):
EOC = log [1 + Eά(λ - 1)]/log λ----- (eqn. 18.46, page 18.17, 6th edition Perry)
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Where Eά/Emv= 1/(1 + EMV [/ (1-)]----- (eqn. 18.27, page 18.13, 6th edition
Perry)
Emv = Murphee Vapor efficiency,
E. = Murphee Vapor efficiency, corrected for recycle effect of liquid entrainment.
(L/G)× {g/l}0.5 = 0.039
for (L/G)×{g/l}0.5 = 0.039 and at 80 % of the flooding value,
We have from fig.18.22, page 18.14, 6th edition Perry
= fractional entrainment, moles/mole gross down flow = 0.076
Eά = Emv 1/[1 + Emv [/ (1- )]]
= 0.81[(1+0.81[0.076/ (1-0.076)])]
⇒Eά= 0.773
Overall Efficiency = EOC = log [1 + Eά(λ- 1)]/log λ
EOC = log [1+ 0.7730(0.76-1)]/log 0.76
Overall Efficiency = EOC = 0.726
Actual trays = Nact = NT/EOC = (ideal trays)/ (overall efficiency)
Where NT = Theoretical plates,
Nact = actual trays
Nact = 17/0.72623.41=25
Thus actual trays in rectifying section = 13
Thus, Actual trays in the Stripping Section = 12
Total Height of distillation column= 25×ts = 25×457 = 11425 mm = 11.425 m
Design of autoclave
INTRODUCTION
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Autoclaves have been used in industry for many decades. As
technology has progressed so has autoclave design, initially from basic riveted
steam heated vessels to vessels fabricated utilizing the latest welding techniques
with highly sophisticated computerized control systems
The industries that make use of autoclaves have also evolved over
the years. Initially used in the textile, timber, food, sterilizing and rubber
industries, autoclaves are now essential items in the advanced composites and
Investment casting industries
Commercial pressure autoclaves have been in operation since mid
1950s. Historical production data suggest that these early autoclaves were
originally designed with excess capacity (Bere zowsky, Collins, Kerfoot, and
Torres, 1991). Process development initially consisted of tests in batch autoclaves.
While in some cases this stage had been followed by continuous pilot plant tests
in multi-compartment autoclaves, in many cases the batch test residence times
were extended to the continuous commercial stage using some factor, resulting in
an over estimation of the required autoclave volume. In some other instances the
continuous stage of testing had not been performed or its results could not be
interpreted as a continuation of batch tests due to differences in ore composition,
grind size and reactor conditions. In such cases, it would be important to be able
to scale-up the batch test data to a multi-compartment continuous mode using a
sound approach that takes into account both the physical and chemical aspects of
the leaching reaction as well as the reactor configuration.
For use in the production of advanced composite materials, a hot
atmosphere autoclave has to achieve the following criteria:
Fail to safety, safety systems
Achieve the required internal environment (ie. heat and pressure)
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Programmable temperature control and uniform temperature distribution
Programmable pressure control
Computerised process control, monitoring and data logging
Not only the autoclaves are made of a no. of different materials,but the
sizea,general design and arrangement for heating are so varied that one might
forgive a chemist,used only to most common forms,for failing to some of the rare
types of modern apparatus as being autoclave at all.
Two particular types of autoclaves have beenselected as covering the most
important ranges of high pressure work.These are
1. High pressure autoclaves
2. Low pressure autoclaves
Autoclaves can be made of Iron,steel,copper bronze or tin but
some kind of the steel is by the far the most common material used in their
manufacture on account of its great strength.Modern practice supports use of
nickel steel and nickel chrome steel for those autoclaves designed to withstand
very high pressure.
Autoclaves are made of very large size,from small labortary pieces
of apparatus of few hundered cubic centimeters capacity to huge pans capable of
holding a charge of twenty thousand gallons.
Autoclaves are generally cylindrical in shape,height being
from 2-3 times the diameter.Bottom may be ellipsoidal or dishad bottom.In order
to give strength all sharp vurves or angles axcluded from design the top of the
autoclave known as cover is fixed on to flanges
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Many of the different materials, are used for packing of
the joints. The commonest packings are lead,copper,aluminium and asbestos.Not
only does the choice of material used on type of autoclave ,but also depend on
pressure.It is more common one type to fix one or more safety valves of the
ordinary steam boiler type to an autoclave.But these were constantly getting
chocked with material either distilled or splashed into the valve.
Indeed, there is no possibility of chemical reaction between the
charge and the material of which autoclave, if it is doubtful the use of liner is
necessity. The liner is a thin walled vessel to fit inside of the autoclave and
intended to hold the charge. The liner may be made of lead, steel tin, copper, iron,
or sometime zinc.
FACTORS AFFECTING AUTOCLAVE SIZE
In a recent paper on design of autoclaves for pressure leaching of
nickel literates (King, 1996), the following have been listed as the determining
factors in the pressure autoclave size: solids throughput and size distribution
1. selected autoclave circuit
2. slurry density
3. retention time
4. temperature
5. number of operating trains
6. shipping restrictions.
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Design Calculations
MASS IN:
NaOH
=
25.958 kg
H2O
=
145.6 kg
Phenol
=
420.82 kg
Na-Phenolale
=
5066.88 kg
CO2
=
2304.64
Total mass
=
7963.89 kg
Design:
Average density of fluid
=
soln = 1080.46 kg/m3
Volume of reactant
=
V = m/soln
=
7.38 m3
V
=
10.332 m3
H/D
=
2.3
VTotal
=
Vcylinder shell + Vellipsoidal head
10.332
=
2
D H
D3
4
24
=
2
D ( 2.3D)
D3
4
24
D
=
1.92 m
H
=
2.3 D
=
4.43 m
40% design allowance
CALCULATIONS FOR AGITATOR
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Type: Turbine Pitched Blade
i)
Diameter of impeller
=
Da = 1.92/3
ii)
Liquid level
=
0.64
iii)
Height of impeller from bottom
=
Ha = 0.64 m
iv)
Impeller blade width
=
Dt
= 0.128 m
15
v)
Four vertical baffles width
=
Dt
= 0.192 m
10
POWER CONSUMPTION
=
Np Nr3 Da5
=
1200 ft/min.
Ts
=
× Da × Nr
1200
=
3.14 × (0.64 × 3.28) × Nr
Nr
=
181.8 r/min.
=
3.03 r/sec.
P
Ts = Tip speed
Re
=
=
D a2 Nr
0.298
44998.18
Re is greater than 10,000 so P is independent of Np and
Np
=
KT
P
=
5
3
KT D a Nr
=
1.65 × 0.645 × 3.033 ×
=
5.325 Kw
=
5.325 × 1.35
1080.46
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
=
7.188 hp
Efficiency of motor 80%, so
Power req.
CALCULATION
OF
INSIDE
FILM
=
7.188
0.8
=
8.98 HP = 9 H.P.
CO-EFFICIENT
FOR
REACTOR
For pitched blade turbine
Cp
K
hi Dt
K
=
0.67
0.62 N Re
Cp (mixture)
=
2.1 KH/Kg-K
(mixture)
=
0.298 Pa-sec.
K (mixture)
=
0.685 W/m2-K
=
0.62
hi
0.000685
0.67 2.1 0.000298
44998.18
1.92
0.000685
0.33
×
0.33
0.586 KJ/m2 – sec – K
=
FILM CO-EFFICIENT OF JACKET
We have steam in jacket so
hj
=
1500 Btu/ft2 – hr – Fo
=
0.0753 KJ/m2 – sec – K
=
n Cp T
=
0.65 × 78 × 100
HEAT BALANCE
Na OH
Q
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
=
5070 KJ
=
8.1 × 75.24 × 100
=
60944.4 KJ
=
44.2 × 243.17 × 100
=
1074811.4 KJ
=
4.48 × 45.7 × 100
=
20473.6 KJ
=
30.62 × 257.76 × 100
=
789261.1 KJ
=
1950560.52 KJ
H2O
Q
Na – ph
Ph
Na – Sal
Total heat req.
Time Req. for Heating
ln
Ts T1
Ts T2
=
UA
(t)
m Cp
ln
235 220
235 120
=
0.0667 26.58
(t)
7963.89 2.1
=
2.76 hrs
=
1950560.52
2.76
=
706724.82 KJ/hr
t
Heat Flow Rate
Q
Mass flow rate of steam
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Q
=
Cp T
m
706724.82
=
× 2.029 × 15
m
m
=
23220.79 kg/hr
=
6.45 kg/sec.
Q
=
U D A LMTD
1
UD
=
1
1
UD
=
For Heat Transfer Area
1
0.586
1
0.0753
0.0055
hi
1
hj
X
K
0.0055
UD
=
0.0667 KJ/m2-S-K
Q
=
U D A LMTD
706724.8
=
0.0667 × A × 170.41
A
=
17.27 m2
=
17.27
26.58
=
65.4 %
Now
Effectiveness of jacket
MECHANICAL DESIGN
SHELL THICKNESS
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
X
=
Pri
Cc
(SE j 0.6 P)
P
=
Pressure of system
ri
=
Radius of shell
S
=
Stress of material
Ej
=
Efficiency of joint
Cs
=
Corrosion rate
=
101.3 7.7 1.292
0.002
160000 0.85 0.6 7.7 101.3
=
5.502 mm
X
HEAD THICKNESS
=
gh
=
1080.46 × 9.8 × 4.43
=
46.907 KPa
Total pressure at base
=
827.697 KPa
t
=
Pi D i
2 S E j 0.2 P
Pressure at the lease
827.69 1.92
2 160000 0.85 0.2 827.69
=
=
5.84 mm
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
ACIDIFICATION TANK
When the reaction in autoclave is completed the outcome stream is
at Temperature of 125 Co and it needs to be cooled to approach the acidification
temperature requirement. So this stream is passed through dilution tank where
along with lowering of temperature to 63 C0, the dilution of feed for reactor is
carried out to get maximum conversion. Then this stream containing reactant
sodium salicylate enters into the “Acidification tank” and also another stream of
40% sulfuric acid is added into the reactor. The reaction is carried out at 20 C 0
because this temperature gives maximum conversion at these conditions of temp.
And pressure. As for as the nature of reaction is concerned, it’s an “Exothermic
reaction”. So the released amount of energy is simultaneously absorbed by the
circulating cooling water in the jacket surrounding the reactor. At the end of the
reaction time when the sodium salicylate is converted into salicylic acid, its in
precipitate form along with sodium sulfate. So this solution is then send to the
“centrifuges” where separation is carried out.
DESIGN CALCULATIONS OF REACTOR.
BATCH REACTOR:
RESIDENCE TIME: 3 hr
NUMBER OF REACTORS
TOTAL CAPACITY OF THE REACTORS: (25 TONS /DAY)
CAPACITY FOR EACH REACTOR:
TOTAL MASS IN PER BATCH:
(8.33 TONS/DAY)
(1.04167 TONS/BATCH)
COMPONENTS IN FEED
MASS INPUT
DENSITY
(Kg)
(Kg/m3)
6.417
2100
VOLUME
(m3)
NaOH
.003
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
H2O
3114.32
997
3.124
Phenol
1.1797
Sodium phen.
1.44
Sodium salicylate
1023.7
0.00115
1259
1216.83
0.00114
1373
0.886
H2SO4
372.78
1834.48
0.203
VOLUME OF REACTOR
(VOLUME =MASS/DENSITY)
TOTAL VOLUME = VNaOH+VH2O+VPhenol+Vsod.phen+Vsod.sal+VH2SO4
=4.2185 m3
DIAMETER OF TANK CALCULATIONS
According to the consideration for reactor diameter calc.
Diameter of tank = Height of liquid in tank
We know the formula for the volume of reactor,
Volume = (∏/4)*DT2*Hl)
(As Hl=DT)
Therefore
DT = 1.75 m
As the volume of reactor is not fully filled and 25% is kept as vacant space,
So,
Total volume of reactor = 4.218/0.75 = 5.624 m3
BOTTOM OF REACTOR:
The bottom of reactor (with reference “liquid mixing and
processing”by Holland and chpman) is taken as Hemispherical. So the total
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
volume now will be contributed by this part of reactor also.
To find the volume of hemispherical part. We have,
Volume = (2/3 *∏* r 3 )
As (DT = 2*r)
So V = 1.36 m3
Now the total volume of reactor
Total volume = Cylindrical + Hemispherical
VT = 4.2185 + 1.36 = 5.5785 m3
The volume of the reactor also includes the vacant space provided above the
surface. That is taken to be 25% of the total, so now the volume is given as,
VT = 5.5785/. 75 = 7.438 m3
LENGTH TO DIAMETER RATIO:
From literature considering length to diameter ratio, L/D =
1.5
So putting in formula,
V = ∏/4 * DT 2*L
V = 7.44 m3, DT = L/1.5, ∏ = 3.14
L = 2.77 m
According to the literature (with reference “liquid mixing and processing”)
DT = Diameter of the tank = 1.7357 m
Dimpeller = DT/3 = 0.5786 m
HI = Impeller height from bottom = Di = 0.5786 m
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Wi = Impeller Blade width = Di / 5 = 0.1157 m
“r” = Impeller blade length = Di / 4 = 0.14465m
Length of impeller blade mounted on shaft = r/2 = 0.0723 m
Liquid height = Diameter of tank = 1.7357 m
No. of baffles ( According to literature) = 4
Baffle width = Wb = DT /10 = 0.17357
Number of turbine impellers :
= WELH/DT = Height of liquid * (Specific gravity) Avg /DT
Hl = 1.7357No. Of turbine impellers = 1.8
Approx. = 2.
Distance between the impellers = 1.2 * DT = 0.694 m
POWER CALCULATIONS
Step 1:
CALCULATING POWER NUMBER:
To see the graph you need to have Reynolds’s number so calculating NRE ?
NRE = ρ D n2 / μ
“ρ” = 1118.69 kg/m3, D = dia. of impeller, n = R.P.M = 160 (using Tip speed from
lit)
μ = 30 cp.
So
NRE = 1.998*106
Then looking at the graph b/w Reynolds number and power no.
NPR = 5
STEP –2:
CALCULATING FROUD NUMBER
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
NFR = (n2 * Di / gc)
“n” = rev / sec = 2.38, Di = impeller dia. = 1.89 ft , gc = 32.2
so we get from here
NFR = 0.40
Calculating exponent term: “m”
“m”= ( a – log NRE )/ b
a = 1, NRE = 1.99*106, b = 40 (values of a and b are from literature and graph)
Therefore the value of Froud no.’s exponent “m” is given as
m = -0.106
STEP 3:
Formula for “Power” calculation
P = {(NP * n3 * Di5 ρ) / gc}*(NFR) m
“
n ( rev/sec) = 2.38, m = -0.106, NPR = 5, ρ = 69.83 lb/ft3, NFR = 0.40
So the power calculated is given by,
P = 4.775 kw
P = 6.41 h.p
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
HEAT OF REACTION IN REACTOR
Over all heat of reaction = Heat of formation of reaction + Heat capacity change
Step one:
HEAT OF FORMATION CALC.
Δ Hf 0 =?
Heat of formation data is given at 25 Co
Δ Hf0 = (HEAT OF FORMATION OF) – (HEAT OF FORMATION OF)
PRODUCTS
REACTANTS
(Σ Hf) REACTANTS = -9.976 *106 KJ
(Σ Hf) PRODUCT = -9.6543* 107 KJ
SO,
Δ Hf0 = 321560.25 KJ
SENSIBLE HEAT CALCULATIONS
{Σ m cp ΔT} reactants + {Σ m cp ΔT }product = ΔH Reaction
{Σ m cp ΔT} reactants = -592839.0984 kJ
{Σ m cp ΔT} product = -74243 kJ
So, sensible heat change = Δ HReaction = -667082.1232 kJ
Δ Hoverall = Δ Hf +Δ HReaction
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Δ Hoverall = -345522 kJ
HEAT TRANSFER COEFFICIENT CALCULATIONS
STEP 1: Jacket side heat transfer coefficient
The following equation is applicable for the jacket side heat transfer
coefficient
hj = 0.023 Cp *G *NRE-0.02 * NPr-0.67 * (μ / μw).14
At 11.5 C0 :
Cpwater = 4.189 kJ/Kg.K = 0.99 Btu/lb. F0
μ water = 0.99 C.P = 2.38 lb/ft.hr
Kwater = 0.590 w/m.k = 0.3468 Btu/hr.ft.F0
Ρwater = 999 kg/m3
Mass flow rate of water:
It can be calculated as,
Q = m Cp Δ T
Q = (calc. through energy balance) = 345522 kJ = 328018.9 Btu
So, calculated mass of water,
“m” = 21867.93 lbs
Now as we has selected the “Half pipe coil jacket” based on dealt
conditions and literature. Here we need to have a Dia of pipe “d1”. Which
will be used in calc. Hydraulic radius,
The standard dimension are given for the half pipe coil jacket, according
to which,
Diameter of the ½ pipe jacket = 3 1/ 2 inch = 0.292 ft
From literature, for “Half pipe coil” hydraulic radius is given as,DH/2
DH = {4 * 1/ 2 (Π / 4) *(d1) 2 } / {d1 (12 in/ft)}
So,
DH = 0.0382 ft
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Now for Reynolds number calc.
NRE = {ρ * DH *U}/μ
OR
NRE = { G*D}/μ
Where G = mass velocity
G = mass / area* time
So, for 3 h process, we calculate mass velocity,
G = 142058.93 lbs/ft2.hr
Hence Reynolds number can be calculated as,
NRE = 2270.565
So at the end putting all these values in the Equation of Heat transfer
coefficient.
“hj” = 0.023 * NRE –0.2 *NPr-0.67 * Cp * G * (μ / μw).14
Hence
hj = 197.270 Btu/hr.ft2.F0
STEP 2:
REACTOR SIDE HEAT TRANSFER COEFFICIENT
For reactor side heat transfer coefficient we had the equation from the
reference of literature,
NUSSALT NO. = hi DT / K = 0.4 * NRE 0.67 * NPr0.33 * (μ / μw). 14
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
As,
NRE = 185520.
NPr = Cp * μ / K
F0
Cpmix = 0.454 Btu/lb.
μ mix = 11.51 lb/ft.hr
Kavg.
Btu/hr.ft.F
0
=
0.065
Therefore,
2
“hi” = 66.59 Btu/hr.ft .F
0
STEP 3:
OVERALL HEAT TRANSFER COEFFICIENT
For overall heat transfer equation we had the equation.
1 / U = 1 / hi + 1 / hj + X / K
U = overall heat transfer coefficient =?
hi = inside film coefficient. = 66.59 Btu/hr.ft2.F0
hj = Jacket side film coefficient = 197.270 Btu/hr.ft2.F0
X = Thickness of vessel wall =9mm = 0.0295 ft
K = Thermal conductivity of vessel wall (mild steel) = 26 Btu/hr.ft.F0
Therefore we can have the value of “U”,
U = 47.13 Btu/hr.ft2.F0 = 268.28 W/m2.k
TIME
CALCULATION
FOR
ISOTHERMAL COOLING MEDIUM
loge { (Tb1 – Tc) / (Tb2 – Tc ) } ={Ui Ai / W Cp } * Δt
Where as,
Δt = Time taken to cool the agitated liquid. (hr) =?
W = Mass of agitated liquid. (lbm) = 10381.316 lbm
JACKETED
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Tb1= Entering Temp. for liquid in Vessel. (F0) = 145 F0
Tb2= Leaving Temp. for liquid in Vessel. (F0) = 68 F0
Tc = Temperature of cooling medium. (F0)Avg.= 52.7 F0
Ui = Overall heat transfer coefficient.(Btu/hr.ft2.F0) = 47.13 Btu/hr.ft2.F0
Ai = Area ft2?
Area = Π * DT * Hl
A = 3.14 * 5.69 * 5.68 = 101.66 ft2
So the time can be calculated for cooling the mass, by putting all above
values in equation.
Δt = 2.15 hr
SPECIFICATION SHEET
IDENTIFICATION:
Item:
Item number:
No. Required:
FUNCTION:
Acidification reactor
A.R.3
03
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
Formation of product, salicylic acid from reaction of sulfuric acid with
sodium salicylate.
OPERATION:
Batch operation
TYPE OF REACTOR:
Jacketed agitated cylindrical vessel
DESIGN DATA
VESSEL:
VOLUME
7.44 m3
TEMPERATURE
PRESSURE
DIAMETER OF VESSEL
HEIGHT
HEIGHT TO DIA. RATIO
VOLUME OF BOTTOM
NO. OF BAFFLES
20 C0
1 atm.
1.75 m
2.77 m
1.5
1.36 m3
04
AGITATOR:
AGITATOR DIA.
0.5786 m
HEIGHT FROM BOTTOM
WIDTH OF TURBINE BLADE
SPEED OF AGITATOR
POWER REQUIRED
0.5786 m
0.1157 m
140 rpm
4.775 kw
JACKET:
HEAT TO BE REMOVED
HEAT TRANSFER AREA
HEAT TRANSFER COEFFICIENT hJ.
OVERALL HEAT TRANSFER COEFFICIENT UD
WATER INLET TEMP.
345522 kj
9.44 m2
1118.52 w/m2.k
268.7 w/m2.k
4 C0
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
WATER OUTLET TEMPERATURE
19 C0
MATERIAL OF CONSTRUCTION: GLASS LINED MILD
STEEL
SOLIDS SUSPENSION
Maintaining solids in suspension within a liquid is another very common
application for fluid agitation equipment. Industrial examples are diverse as
suspending ore in cyanide leach systems or diatomaceous earth in a filter aide
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
slurry. The nature of the solids, the concentration of solids, and the degree of
suspension desired all contribute to agitator design. In order for solids to be
suspended the impeller must create enough localized flow to over come the
settling velocity of the material to be suspended.
High efficiency axial flow impellers (HF218) and the flow pattern they produce are
best suited for suspending solids.
The degree of suspension is typically broken down into three different categories
in order of increasing difficulty.
On-Bottom Motion—all particles moving on the bottom, with the lighter ones
suspended off the bottom.
Off-Bottom Suspension—all particles suspended off the bottom, but not
uniformly.
Complete Uniformity—all particles suspended uniformly throughout the tank.
The process variables that can be controlled in this category include dissolution
rate for soluble solids, yields (crystallizers) and mass transfer.
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
DESIGN OF CENTRIFUGE
1.
Calculations of Volume of Centrifuge
Volume of centrifuge
2.
3.
5
(1.22) = 1.525 m3
4
=
Calculations of Diameter & height
2
D ×H
4
V
=
H/D
=
0.625
D3
=
1.525 4
3.14 0.625
D
=
1.46 m
&
H
=
0.9125 m
=
0.73 m
Thickness of Cake
R
=
1.46
2
msolid
=
1041.67 + 556.47 = 1598.14
solid
=
2059.3 kg/m3
Vsolid
=
1598.14
= 0.78 m3
2059.3
Vsolid
=
× H (R2 – IRC2)
0.78
=
3.14 × 0.9125 (0.732 – IRC2)
0.78
=
1.53 – 2.86 IRC2
IRC
=
1.53 0.78
2.86
=
Thickness of cake
0.52 m
=
R – IRC
Chapter 1
4.
5.
O-Hydroxy Benzoic Acid and Related Compounds
0.73 – 0.52
=
0.21 m
=
21 cm
Calculation of Inner Radius of Slurry (IRS)
Volume of slurry
=
IRS
=
× H (R2 – IRS2)
(3.14 0.9125 0.73 2 ) 1.22
3.14 0.9125
=
0.33 m
=
33 cm
Relative Centrifugal Force (RCF)
RCF
6.
=
=
0.000142 n2D
=
0.000142 (1000)2 × 4.79
=
680.18 g
=
1
2 ( R 2 IRS 2 )
2
Centrifugal Pressure
Pc
=
1
12.93.5 (104.67) 2 (0.73 2 0.33 2 )
2
7.
=
3.004 × 106 N.m2
=
R/S (Pc + m SR 2)
Wall Thickness
f
1.41 × 108 S
=
(3.004×10 +8037.86×5×0.73×104.672)
0.73
6
2.41 × 108 S
=
2.19 × 106 + 0.64 × 108 s
(2.41 – 0.64)108 S
=
2.19 × 106
Chapter 1
O-Hydroxy Benzoic Acid and Related Compounds
S
8.
=
2.19 10 6
1.77 10 8
=
0.1237 × 10-3 m
=
12.37 mm
=
13 mm
Self and total Stresses
SS
ST
Allowable stresses are
(ST < f)
=
2 R2 m
=
(104.67)2 (0.73)2 (8037.86)
=
4.69 × 107 N/m2
=
2 × 4.69 × 107 N/m2
=
2 × 4.69 × 107 N/m2
=
9.38 × 107 N/m2
=
2.41 × 108 N/m2
=
(Justified)
=
9.
No. of Holes in Wall of Basket
Diameter of hole
=
5 mm = 0.19685”
Area for one hole
=
(0.7874)2
=
0.0004 m2
=
DH
=
3.14 × 1.46 × 0.9125
=
4.18 m2
No. of holes
=
4.18
= 10450 holes
0.0004
No. of holes/m2
=
2500
Total Area available
Chapter 5
10.
Design of Equipments
Power Calculations
Moment of inertia of feed
Where
So,
=
IF =
m1
(R 2 R 12 )
2
R
=
Radius of Basket
R1
=
R – e (e is thickness of liquid)
R1
=
0.73 – 0.115 (0.73)
=
0.64 m
=
1572.89
(0.73 2 0.64 2 )
2
=
96.97 kg.m2
IF
Moment of inertia of basket =
Ib = m2 R2
Where
m2
=
Mass of Basket
m2
=
D H S m
=
3.14 × 1.46 × 0.9125 × 0.01237 ×
=
415.9 kg
Ib
=
415.9 × 0.732 = 221.63
I
=
IF + Ib = 96.97 + 221.63
=
318.6 Kg m2
=
I
=
318.6 ×
=
×
=
111.16 × 104.67
=
11635.12 Watts
=
11.63 Kw
=
15.59 H.P.
8037.86
p
d
dt
104.67
= 111.16 N-m
5 60
Chapter 5
Design of Equipments
=
16. H.P.
____
SPECIFICATION SHEET
1.
Equipment
Centrifuge
2.
Type
Top suspended basket
3.
Method of operation
Batch wise
4.
No. Required
Three
5.
Function
To separate Salicylic Acid crystals
from mother liquor.
6.
Basket Diameter
1.46 m
7.
Speed
1000 rpm
8.
Maximum centrifugal force
680.18 g.
9.
Typical motor size
16 H.P.
10.
Material of construction of basket
Stainless steel 316
11.
No. of holes per sq.meter
2500
Chapter 5
Design of Equipments
Design of dryer
DRYING OPERATION
Drying of solids means the removal of relatively small amounts of water
or other liquid from the solid material to reduce the content of residual liquid to
an acceptably low value. Drying is usually the final step in a series of operation
and the product from a dryer is often ready for final packaging.
Classification of Dryer Types
A wide variety of dryers are used in the process industries. However
following criteria is employed to classify dryers:
1.
Method of Operation
Table 5.7.1 classifies dryer by method of operation. The first subdivision
is by method of heat transfer.
a)
Conduction heating
b)
Convection heating
c)
Infra-red heating, i.e. all forms of radiation heating
d)
Dielectric heating
Distinction sometimes blurred
Freeze drying may be classified as a special case of conduction heating.
The next main sub-division is by type of drying vessel – tray, rotating
drum, fluidized bed, pneumatic, spray. The table also shows which types of vessel
are more readily adapted to operation under vacuum or with inert atmospheres.
Fig. 5.7.1 shows diagrams of most of these types of dryer.
Chapter 5
Design of Equipments
Table 6.1: Classification of Dryers based on Method of Operation
2.
Physical Form of Feed
It must first be emphasized that purely mechanical means should be used
to reduce the moisture content of the wet feed to as low a figure as possible,
because with few exceptions, processes such as evaporation, filtration and
centrifuging are cheaper and faster than equivalent processes in drying plant.
Table 5.7.2 classifies types of dryer in terms of the physical form of the
material to be fed to the plant.
Raw pastes and sludges are difficult to handle into dryers and the drying
rate quickly slows down through the formation of superficial skins having a low
permeability to vapour. This form of feed therefore requires pretreatment by
‘preforming’ into pellets about 5 mm cube or by forming granules with mixedback fines.
Chapter 5
Design of Equipments
Table 5.7.2 Classification of dryers based on physical form of feed
3.
Scale of Operation
Table 5.7.3 shows the types of available dryer classified according to
scale of production. It will be seen that the number of types for continuous large
scale drying is much more limited than for medium scale outputs.
Chapter 5
Design of Equipments
Table 5.7.3 Classification of dryers by scale of production
4.
Nature of the Feed
We then come to classification in terms of overriding features such as
toxic or heat sensitive materials, special form of dry product etc.; these are shown
in Table 5.7.4.
Table 5.7.4 Classification of dryers by suitability for special features
5.
Capital and Operating Costs
Dryers may also be classified in terms of the labour, heat, flexibility and
capital cost of their operation. Mechanical maintenance cost can only be estimated
by study of the designs provided by individual manufacturers.
Chapter 5
6.2
Design of Equipments
SELECTION OF DRYER
When selecting a dryer, there are several questions that need to be
answered for all types of dryers. Rotary dryers will be used to illustrate problems
because they dry more material than any other dryer. A few of the problems are as
follows.
1.
Type of Feed
What type of dryers can handle the feed? If the feed is liquid, dryers such
as spray, drum, or one of the many special dryers that can be adapted to liquids
may be used. If the feed is quite sticky, it may be necessary to recycle much of the
product in order to use a certain type of dryer. The best solution to the feed
problem is to try the material in a pilot unit. The pilot unit for a spray dryer needs
to be near the size of the production unit as scale-up is quite difficult in this case.
2.
Reliability of Dryer
Is the dryer reliable? Is the dryer likely to cause shutdowns of the plant,
and what performance history does this unit have in other installations? How long
is the average life of this type of dryer?
3.
Energy Efficiency
How energy-efficient is this type of dryer? For example, a steam tube
dryer may have an efficiency of 85% while a plain tube type of rotary dryer may
have an efficiency of only 50%. However, production of the steam entails
additional costs so the plain tube may be more efficient in overall production.
Chapter 5
Design of Equipments
The higher the temperature of inlet gas stream, the higher the efficiency
of the dryer in general. A fluid-bed dryer has a high back-mix of gas so it is
possible to use a fairly high entering gas temperature.
Any dryer can use recycled stack gas to lower the inlet gas temperature
and thus obtain a high efficiency for the dryer. However, if there is any organic
material in the stack gas, it may be cracked to form a very fine carbonaceous
particulate which is almost impossible to remove from the stack. Recycle also
increases the dew point of the incoming gas which lowers the drying potential of
the dryer. This lowering of the potential is quite important when drying heatsensitive material. (TD has to have a low value.)
4.
Heating Fuel
What type of fuel can be used for heating? Direct heating is usually the
most efficient unit, and natural gas and L.P.G. are the best fuels. However, both
gases are getting more expensive and in many cases will not be available. The
next best fuel is light fuel oil which can be burned readily with a “clean” stack.
This material is expensive, and in some cases may be in short supply. The third
best fuel is heavy fuel oil which is usually available, but this oil requires special
burners and may not give a sufficiently clean stack. Coal is dusty and hard to
handle. The stack gas usually is too contaminated for use in most installations.
5.
Dust Problem
Does the dryer have a dust problem? Steam tube units use very low air
flow and have minor dust problems, while a plain tube uses high air rates and may
have serious dust problems. In some cases the stack dust removal devices may
cost more than the dryer.
6.
Sensitivity of Material
How heat sensitive is the material to be dried? Most materials have a
maximum temperature that can be used without the product deteriorating. This
temperature is a function of the time of exposure as the thermal deterioration
Chapter 5
Design of Equipments
usually is a rate phenomena. Wet material can stand much higher temperatures in
the gas due to the evaporation cooling.
As an example: A rotary dryer working with alfalfa can use 1400 oF
entering gas in a co-current unit. A countercurrent unit at this temperature would
burn the alfalfa. As the temperature of the entering gas determines the efficiency
of the dryer, co-current dryers, on the average, are more efficient than
countercurrent dryers.
7.
Quality of Product
What quality of product will be obtained from the dryer? Freeze drying
usually will give an excellent product, but the cost is prohibitive in most cases. A
dryer needs to balance quality against cost of production of a satisfactory product.
8.
Space Limitations
What space limitations are placed on the installation? There are certain
height limitations in some buildings, and floor space may be limited or costly.
9.
Maintenance Costs
Maintenance costs are often a major consideration. If moving parts either
wear out or break down due to material “balling-up” or sticking, the plant may be
shut down for repair, and repairs cost money. If this is a problem, a record should
be kept of the performance of the unit. It may be possible to get this information
from a plant which is using this particular unit on a similar product.
10.
Labour Cost
What is the labor cost? A tray dryer has high labor costs, but it is the best
dryer in many cases where only small amounts of material need to be handled.
11.
Availability of Data
Is a pilot unit available which can be used to get data to design the
needed production facility? Nearly all new products need pilot plant data for a
satisfactory design of a dryer. In the case of spray drying, an industrial size unit
needs to be used. Drum and rotary units and most other dryers can be scaled-up
with sufficient success from laboratory sized units.
Chapter 5
Design of Equipments
12.
Capital Investment Needed for Accessories
What is the capital investment for the dryer and all the accessories?
13.
Power Requirement
What is the power requirement for the dryer? A deep fluid-bed dryer
needs hot gas at a higher pressure than most other dryers: 1000 ft3/min of gas
requires approximately 1 hp per 4 in. of water pressure.
14.
Scale of Production
What quantity of product is desired? For larger production a spray or
rotary dryer should be considered. Rotary and spray dryers handle most large
production demands, but in small production plants other dryers are often more
economical.
15.
Rangeability
Can the dryer perform over a wide range of production rates and still
give a satisfactory product in an efficient manner?
Once the above points have been examined, it is possible to select a few
types of dryers that appear to be the best for the particular operation. Pilot plant
data should be obtained on these dryers to determine the size needed. Firm
quotations should be obtained from the manufacturers. The most economical
dryer now can be selected on the basis of quality of product and capital and
operating costs.
Chapter 5
5.7.3
Design of Equipments
WHY TO SELECT HORIZONTAL ROTARY VACUUM
DRYER
There are following reasons in order to select horizontal rotary vacuum
dryer for our process.
1.
Because the material coming from centrifuge is sticky, so in order to
make an effective heat transfer, surface renewal is needed. Because
materials stick to the surface of dryer so an agitator is required for
surface renewal and to make an effective heat transfer.
2.
Salicylic acid has tendency to disintegrate into benzoic acid and phenol
at its boiling point, therefore vacuum is employed in order to lower the
boiling point of water.
Drying Operation
The centrifugalize undried product salicylic acid is weighed, then by
means of the hoist, lifted to the hopper of the cylindrical vacuum drier, at the
same time. The stirrer inside the drier is started up.
After loading, 0.4 MPa & 145oC steam is used in the jacket for heating.
The vacuum pump is started up, the system is at vacuum state, vacuity 0.08
MPa, the material temperature should not exceed 70oC during the drying process.
The drying time is usually 3 ~ 4 hours. After drying samples are taken and
analyzed. When the moisture is 0.5%, the product will be qualified and ready for
discharge. The obtained product is coarse salicylic acid, weighed, then transported
to the sublimation refinement.
The power dust of salicylic acid produced during drying process will be
returned to the cylindrical vacuum dryer by the spiral conveyor; small amount of
powder dust will be drawn into the phenol water receiver through the water tank,
Chapter 5
Design of Equipments
and then pushed under pressure into the centrifuge for recovery of SA (Salicylic
acid).
The condensed water produced in the drying process, flows by gravity
through the condenser to the phenol water receiver, from where it is pushed by
pressure into the centrifuge, the phenol water flows into the overflow pool, then
into the sewage pool for recovery of phenol by extraction.
5.7.4
DESIGN
OF
HORIZONTAL
VACUUM
ROTARY
DRYER
5.7.4.1 Data for Calculation of Horizontal Vacuum Rotary Dryer
Amount of feed
=
ms = 45660 kg/day
Moisture content in feed
=
X1 = 16% (7308 kg/day)
Feed temperature, ti
=
293oK
Moisture content in product
=
X2 = 0.19% (72.98 kg/day)
Product temperature, to
=
343oK
Solids specific heat, Cs
=
Cs
=
[0.2663 (160.9)]S.A. + [0.1376 (137.29)]Na2SO4
=
61.74 KJ/kg mol oK
Water specific heat
=
75 KJ/kg mol oK
Heating medium
=
Steam
Steam temperature, T
=
418 oK at 0.4 MPa
Enthalpy of steam
=
2739 KJ/kg
5.7.4.2 Thermal Data for Moisture and solids
Evaporation enthalpy
Water
enthalpy
at 15 KPa (vacuum)
at
=
2370 KJ/kg
=
(327oK)
(B.pt.)
225.622 KJ/kg
54oC
Chapter 5
Design of Equipments
Water
Average (54oC – 145oC)
(327oK – 418oK)
vapor
Solids temp. in constant rate during period
Product
Moisture
rate period, Xc
at
rate, 101
100
2669.78
=
327oK
start
=
1
Heating
Heat
transfer
KJ
hr.m 2 .K o
Surface loading, %
=
enthalpy,
KJ/kg
of
falling-
8%
2
Cons tan t rate
Drying
3
Falling rate
Drying
585
300
80
60
Calculation
Dry solids
=
m s d = ms (1 – X1)
=
45660 (1 – 0.16)
=
38354.4 kg/day
Product, m s
=
m sd
38354.4
38425 kg / day
(1 X 2 ) (1 0.0019)
p
Moisture
falling-rate
at
=
Moisture in final product =
change
to
m sd X c
38354.4 0.08
(1` X c )
(1 0.08)
3335 kg
=
38425 x 0.0019
= 73 kg/day
=
ms – msp
= 45660 – 38425
Evaporation
Total evaporation
= 7235 kg/day
Chapter 5
Design of Equipments
Evaporation upto falling-rate
= 3335 – 73
Zone
= 3262 kg/day
Evaporation upto constant-rate
= 7235 – 3262
Zone
= 3973 kg/day
Chapter 5
Design of Equipments
Zone
Operation
1
2
3
Heating
Constant-rate
drying
Falling-rate drying
Temperature, oK
Steam temp. oK
418
418
418
418
418
Solids temp. oK
293
327
327
327
343
125
91
91
91
75
Tm
Log mean temp. oK (Tm)
difference
(T t o ) (T t i )
ln[(T t o ) / (T t i )]
107.102
91
82.74
Temp.diff (oK)
Heat Loads, KJ/hr
Solid heat load (KJ/hr)
Day
kg-mol
Cp (Heat Capacity) in KJ
24 hr
Day
Kg mol oK
274.88x (327 293) x 61.74
24
= 24,042 KJ/hr
Liquid heat load
heat
406 x (327 293) x 4.191
24
load
Zone totals of heat load
(KJ/hr),
Qh, Qc, Qt
Surface
area,
m2
(adjusted for loading)
=
Qh
Tm h . h h . L n
4.5(343 327) x 4.191
24
= 12.57 KJ/hr
26,453
274.88x (343 327) x 61.74
24
= 11,314 KJ/hr
= 2410.52 KJ/hr
Evaporation
(KJ/hr)
3973x 2370 3262( 2609.78 225.622)
24
24
=392,334
KJ/hr
= 332,202 KJ/hr
= 392,334
= 34,3528
=
Qc
Tm c . h c . Lc
=
Qf
Tm f . h f . L f
Chapter 5
Design of Equipments
=
26453
107.102 101 1.0
= 2.44 m2
=
343528
=
392334
82.74 300 0.6
91 585 0.8
= 9.21 m2
= 23.07 m2
Chapter 5
Design of Equipments
Total heat surface required, m2, A
=
34.72 m2
Total heat load, KJ/hr, QT
=
762,316 KJ/hr
=
762316
2739
=
278.32 kg/hr
Steam flow-rate
=
QT
HS
From Table of sizes ……. Vacuum rotary dryer
For heating surface of 34.72 m2,
Total capacity of dryer, V
Let
14.036 m3
=
6
=
= D2L
4
14.036
=
(D2) (6D)
4
2.979
=
D3
D
=
1.44 m
L
=
6D = 6 × 1.44 = 8.63
Volume of cylindrical dryer, V
m
L/D
=
Chapter 5
Design of Equipments
v
Rate of drying, m
=
h y (T Ti ) A
i
v
m
=
Rate of drying (kg/hr)
T
=
temperature of steam
Ti
=
Temperature at interface
A
=
Drying area
i
=
Latent heat at temperature, Ti
hy
=
Heat transfer co-efficient, KJ/hr.m2.oK
v
m
=
585 ( 418 383) 34.7
2229.94
v
m
=
318.61 kg/hr
tT
=
ms
X
X1 X c X c ln c
v
m
X2
=
7235
0.16 0.08 0.08 ln 0.08
318.61
0.0019
=
8.61 hr
tT
For total drying time the # of batches are calculated as:
No. of batches
=
24
tT
=
24
2.79
8.61
3 batches
DESIGN OF STEAM JACKET
From Perrys’ Chemical Engineers’ Handbook
For steam in Annulus:
Do
D i
Chapter 5
Design of Equipments
As
DI
Do
= 0.664
Di
= 1.44 m (calculated)
So, Do
Do
=
1.44
0.664
= 2.17 m
Hence Annulus dia for steam flow
Da
Area of annulus
=
Do – Di
=
2.17 – 1.44
=
0.73 m
=
(D a ) L
4
=
(0.73) 8.63
4
=
4.95 m2
Chapter 5
Design of Equipments
SUBLIMATION
“Sublimation is a process in which a solid is vaporized without
going into liquid state and then are condensed back into solid state.”
Where as:
Quasi-sublimation:
“In this process a molten solid is vaporized and then condensed
directly back to solid state.
Normally a substance processed by sublimation, is a solid at or
near ambient temperature and pressure and has a reasonably high vapors pressure
at operating temperatures, typically a minimum of 5 μm.Hg absolute.
Reviews of industrial applications of sublimation techniques
have been made by: “Kemp” (1958); “Holden and Bryant” (1969); “Mellor”
(1978); “Kudela and Sampson” (1986);
Sublimation is used to separate volatile solids and to purify the
substances. One should consider using sublimation when performing the
following steps:
Removing the volatile product from a non-volatile material or mixture of
materials or removing the volatile materials from a non-volatile product.
Separating more volatile from less volatile components in a mixture of
volatile materials.
Separating a product from its impurities when either is unstable or heat
sensitive at temperatures close to the melting point.
Chapter 5
Design of Equipments
Preparing a required type or size of crystalline product.
Purifying a wet feedstock in which drying and purification can take place
in a single sublimation step.
Some commercial sublimation processes are given in following table:
TABLE: VARIETY OF SUBSTANCES CAN BE SUBLIMED
ALUMINIUM CHLORIDE
MOLYBDENUM TRIOXIDE
ANTHRACINE
NAPHTHLENE
ANTHRANILIC ACID
b-NAPHTHOL
ANTHRAQUINONE
PHTHALIC ANHYDRIDE
BENZANTHRONE
O-PHTHALIMIDE
BENZOIC ACID
PYROGALLOL
CALCIUM
“SALICYLIC ACID”
CAMPHOR
SULFURE
CHROMIUM CHLORIDE
TEREPHTHALIC ACID
FERRIC CHLORIDE
TITANIUM CHLORIDE
ICE
THYMOL
IODINE
URANIUMHEXAFLUORIDE
MAGNESIUM
ZIRCONIUM
TETRACHLORIDE
Chapter 5
Design of Equipments
PRINCIPLES OF OPERATION:
The basic principles of vaporization and
condensation have been discussed by “Runter”, “Goldfinger”, and “Hirth”, in
1964. By “Strickland-constable” in 1968 also by “Mellor” in 1978.
The mechanism of a sublimation process can be described with reference
to the “Pressure Temperature “phase diagram in the given figure. The significance
of the P-T diagram applied to one component system. The phase diagram is
divided into three regions, Solid, Liquid and vapor, by the sublimation,
vaporization and fusion curves.
These three curves intersect at a unit point called “Triple point.
The position of the triple point in the diagram is of utmost importance
If it occurs at a pressure above atmospheric, the solid cannot melt
under normal atmospheric conditions, and process is called as
“True Sublimation” :( Direct from solid to vapour).
If it occurs at a pressure less than atmospheric, however, certain
precaution are necessary, “The phase changes Solid to vapor” or
“vapor to solid” must be controlled, because it may involve the
“liquid state”.
In industrial applications it is not uncommon for liquification to be
allowed in “vaporization stage”, to facilitate better heat transfer, but this must
Chapter 5
Design of Equipments
never be allowed in the “Desublimation or Crystallization step”. The condensation
equipment therefore must operate well below the triple point. If liquification is
employed before vaporization, the operation is often called “PseudoSublimation”.
Both true and Pseudo-sublimation are depicted in the fig. For the case of a
substance with a triple point at a pressure greater than atmospheric, “TRUE
SUBLIMATION” occurs. The complete cycle is given by the path “ABCDE”,
the original solid A is heated to some temperature represented by B. The increase
in the vapor pressure pressure of the substance is traced along the sublimation
curve from A to B. The condensation side of the process is represented by the
broken line “BCDE”. As the vapor passes out of the vaporizer into the condenser,
it may cool slightly and it may become diluted as it mixes with some inert gas
such as air. Point C, therefore representing a temperature and partial pressure
slightly lowers than point B.
The condensation side of the process is represented by the broken line
“BCDE”. As the vapors pass out of the vaporizer into the condenser, it may cool
slightly, and it may become diluted as it mixes with some inert gas such as Air.
POINT C, therefore representing a temperature and partial pressure slightly lower
than point B, can be taken as condition at the inlet to the condenser. After entering
the condenser the vapor mixes with more inert gas and partial pressure of the
substance and temperature will drop to some point D. Thereafter the vapor cools
essentially at a constant pressure to the conditions represented by point E, the
temperature of the condenser.
When the Triple point of the substance occurs at a pressure less than
atmospheric, the heating of the solid may easily result in its temperature and
vapor pressure exceeding the Triple point conditions. The solid will then melt in
Chapter 5
Design of Equipments
the vaporizer through path A to B’ in the fig. represents such a process. How ever
great care must be taken in the condensation stage. The partial pressure of the
substance in the vapor stream entering the condenser must be reduced below the
Triple point pressure to prevent initial condensation to a liquid. The required
partial pressure reduction can be brought about by diluting the vapors with an
inert gas, but the fractional pressure drop in the vapor lines is generally sufficient
in itself. Point C represents the conditions at the point of entry into the condenser
and the condensation path is represented by C’DE.
PROCESSE AND EQUIPMENT:
On commercial scale, it is difficult to vaporize and
condense a solid as well as to control these steps. Heat transfer is typically low
and removing condensed solids from a surface is not possible. A proposed
sublimation process can become commercial if economical solutions to such
problems can be found.
Sublimation techniques can be classified conveniently into three basic types:
SIMPLE SUBLIMATION
VACUUM SUBLIMATION
ENTRAINER SUBLIMATION
Chapter 5
Design of Equipments
SIMPLE SUBLIMATION:
In this type of sublimation the solid material is heated and
vaporized, and then vapor diffuses to condenser. The driving force for diffusion is
the “partial pressure difference” between the vaporizing and condensing surfaces.
The vapor path between the vaporizer and the condenser should be as short as
possible to reduce the resistance to flow. Simple sublimation has been practiced
for the, Ammonium chloride, iodine and for the flowers of sulfur also.
VACUUM SUBLIMATION:
It’s a natural follow on from simple sublimation. The
transfer of
Vapor from vaporizer to the condenser is enhanced by reducing the pressure in the
condenser, which thus increases the partial pressure driving force. Iodine,
pyrogellol and many metals have been purified by this type of sublimation. The
exit gases from the condenser usually pass through a cyclone or a scrubber to
protect the vacuum-raising equipment and to minimize the loss of product.
ENTRAINER SUBLIMATION:
In this type of sublimation an inert gas is blown into the
vaporization chamber of a sublimer to increase the rate of flow of vapors to the
condensing equipment and thus increase the yield. Such a process is known as
“ENTRAINER SUBLIMATION”.
The use of entrainer in process has many desirable features:
It enhances the vapor flow from sublimer to the condenser
It also provides the heat needed for sublimation
Providing an efficient mean to control the temperature
Chapter 5
Design of Equipments
Providing enough contact time to saturate air with vapor
The purification of Salicylic acid provides a good
application of the use industrially of entrainer sublimation. Air may be used as the
carrier gas: But as salicylic acid can be decarboxylated in hot air, a mixture of air
and carbon dioxide is often preferred. A 5 to 10% of carbon dioxide is recycled
through the plant, passing over heater coils before over the containers, e.g. Bin or
trays, holding the impure salicylic acid in the vaporizer. The vapors are then
passed through the series of air cooled chambers, where sublimed salicylic acid is
deposited. A trap removes any entrained sublimate before the gas stream is
returned to the heaters. Make up CO2 and air are introduced into the system as
required, and the process continues until the containers are emptied of all volatile
matter.
The impure material is pulverized in a mill, and hot air or
any suitable gas mixture blows the fine particles, which readily volatilize, into a
series of separator, e.g. cyclones, where non-volatile solid impurities are removed:
A filter may also be fitted in the vapor lines to remove final traces of impurities.
The vapors are then passed to the series of condensers. The gases can be recycled
or passed to atmosphere through a cyclone or scrubber.
To obtain the max yield of the product the air should be saturated with the
vapors of salicylic acid at 150 0C and saturation will only be approached if the air
and salicylic acid are contacted for a sufficient period of time at the required
temperature. A fluidized bed vaporizer may allows these optimum conditions to
be approached; but if air is simply blown over bins or trays is containing the solid,
saturation will not be achieved and the actual rate of sublimation will be less than
that calculated. In some cases the degree of saturation may be as low as 10 % of
the possible value.
Chapter 5
Design of Equipments
The calculated loss of product in the condenser exit gases is only a
minimum value. Any other losses due to solids entrainment will depend on the
design of the condenser and cannot be calculated theoretically. An efficient exit
gas scrubber can, of course, minimize these losses.
Chapter 5
Design of Equipments
Chapter No. 6
Instrumentation and Process Control
Chapter 5
Design of Equipments
Chapter 6
Instrumentation and Process Control
Measurement is a fundamental requisite to process control. Either the
control can be affected automatically, semi automatically or manually. The quality
of control obtainable also bears a relationship to accuracy, re product ability and
reliability of measurement methods, which are employed. There fore , selection of
the most affect means of measurements is an important first step in design and
formulation of any process control system.
Temperature measurement and control
Temperature measurement is used to control the temperature of outlet
and inlet streams in heat exchangers, reactors, etc.
Most temperature measurements in the industry are made by means of
thermocouple to facilitate bringing the measurements to centralized location. For
local measurements at the equipment bimetallic or filled system thermometers
are used to a lesser extent. usually, for high measurement accuracy, resistance
thermometers are used.
all these measurements are installed with thermo wells when used locally. This
provides protection against atmosphere and other physical elements.
Pressure measurement and control
Like temperature pressure is a value able indication of material state
and composition.
In fact, these two measurements considered together are the primary evaluating
devices of industrial materials.
Pumps, compressers and other process equipment associated with pressure
changes in the process material are furnished with pressure measuring devices.
Chapter 5
Design of Equipments
Thus pressure measurement becomes an indication of an energy decrease or
increase.
Most pressure in industry are elastic element devices, either directly connected for
local use or transmission type to centralized location. Most extensively used
industrial pressure is the Bourderi Tube or a Diaphram or Bellow gauges.
Flow measurement and control
Flow indicator are used to control the amount of liquid. Also all
manually set streams require some flow indication or some easy means for
occasional sample measurement.
For accounting purposes, feed and product streams or metered. In addition
utilities to individual and grouped equipment are also metered.
Most flow measures in the industry are/ by Variable Head devices. To a lesser
extent variable area is used as are many types available as special metering
situation arise.
Control scheme of distiallation column
General consideration
Objectives
In distillation column any of following may be the goals to achieve.
1. Overhead composition
2. bottom composition
3. Constant over head product rate.
4. Constant bottom product rate.
Manipulated variables
Any one or any combination of following may be the manipulated
variables.
1. Steam flow rate to reboiler
2. reflux rate.
Chapter 5
Design of Equipments
3. Overhead product with drawn rate.
4. Bottom product withdrawn rate.
5. Water flow rate to condenser.
Loads or disturbances
Following are typical disturbances.
1. Flow rate of feed.
2. Composition of feed.
3. Temperature of feed.
4. Pressure drop of steam across reboiler.
5. Inlet temperature of water for condenser.
Control scheme
Overall product rate is fixed and any change in feed must be absorbed
by changing bottom product rate. The change in product rate is accomplished by
direct level control of reboiler if the stream rate is fixed feed rate increases then
vapour rate is approximately is constant and the internal reflux flow must
increase.
Advantage
Since and increase in feed rate increases reflux rate with vapour rate
being approximately constant, then purity of top product increases.
Disadvantage
The overhead change depends on dynamics of level control system that
adjusts it.
Chapter 5
Design of Equipments
Chapter 5
Design of Equipments
Chapter No. 7
BASIC PRINCIPLES OF
HAZOP STUDY
Chapter 5
Design of Equipments
Chapter 7
BASIC PRINCIPLES OF HAZOP STUDY
The basic concept of the hazard and operability study is to take a full
description of process and to question every part of it to discover what deviation
from the intention of the design can occure and what can be their causes and
consequences.
The seven guide words recommended in the chemical industries
association(CIA) booklet are used.In addition to these words,the following words
are also used with precise meaning.
Intention
Deviation
Causes
Consequences
Hazards
In HAZOP study,each segment(pipeline,piece of
equipment,instrument,etc) is carefully examined and all possible deviations from
normal operating conditions are spesified.Some of guide words works
recommended by CIA are given in the following Table.
GUIDE WORDS
No or Not
No part of the intent is achieved & nothing else
occurs
(e.g. no flow)
More
A quntitatibe increase(e.g. flow rates & temprature)
Loss
A quntitatibe decrease(e.g. low pressure)
As well as
A qualitative increase (e.g. an impurity)
Part of
A qualitative decrease (e.g. only one of two
components in a mixture)
Chapter 5
Design of Equipments
Reverse
Opposite (e.g. block flow),the elogical opposite of
Other than
No part of the intent is achieved & something
the intention
completely different occurs (e.g.flow of wrong material.)
These guide words are applied to flow,temperature,pressure,liquid
level,composition and any other variables affecting the process.The consequences
of these deviations on the process are then assessed and the measures needed to
detect and correct deviations are established.
TYPES OF OPERATING DEVIATIONS
Major operating limits
Flow
Temperature
Time
Contact time
Sequence
Pressure
Design cycle
Level
Local effects
Chemical reactivity
Distribution
Mechanical stresses
Mixing
Other operating limits
Hotspots
Corrosion
Overheads
Erosion
Resonance
Resistance
Stressing
bearings/shafts
Fouling
Lubricating faults
Cavitation
Blockage
Vibrations
Failure to contain
material
Loading
Spillage
Expension
Leakage
Contraction
Vented material
Cycles of activities
Construction
Chapter 5
Design of Equipments
Environmental factors
Deffective
materials of construction
Viscosity
Plant
Miscibility
Equipment
incomplete
unsupported
Melting/Boiling point
Equipment
Density
Equipment
not aligned
not tight
Vapor density
Major
deviations
Phase
Start up/shut
down
Appearance
Particle size
Maintnance
Planned
changes in normal operations
Chemical composition
Hazardous materials
Others
Supply/Equipment failure
Demand changes
Unplanned ignition source
Reactions
Extent type
Control distribution
Loss of
communication
Side reaction
Catalyst behavior
Human error
Climate effects
1. activity
2. Toxicity
Chapter 5
Design of Equipments
In the scheme shown in the following figure soda is unloaded from
tank trunks into a storage tank and is transferred to the mixing tank.HAZOP study
on a storage is briefly presented in the table.
Chapter 9
Cost Estimation
Chapter No. 8
Potential Health Effects
Chapter 9
Cost Estimation
Chapter 8
Potential Health Effects
Inhalation:
May cause irritation to the respiratory tract. Symptoms may include
coughing, sore throat, labored breathing, and chest pain.
Ingestion:
Ingestion of sizable amounts can cause "salicylism", as evidenced by
abdominal pain, vomiting, increased respiration, and mental disturbances.
Fatalities resulting from respiratory or cardiovascular failure are known.
Mean lethal adult dose of salicylates is between 20 and 30 grams.
Skin Contact:
May cause irritation with redness and pain.
Eye Contact:
May cause irritation, redness and pain.
First Aid Measures
Inhalation:
Remove to fresh air. Get medical attention for any breathing difficulty.
Ingestion:
Induce vomiting immediately as directed by medical personnel. Never give
anything by mouth to an unconscious person. Get medical attention.
Skin Contact:
Immediately flush skin with plenty of water for at least 15 minutes. Remove
contaminated clothing and shoes. Wash clothing before reuse. Thoroughly clean
shoes before reuse. Get medical attention if irritation develops.
Eye Contact:
Immediately flush eyes with plenty of water for at least 15 minutes, lifting upper
Chapter 9
Cost Estimation
and lower eyelids occasionally. Get medical attention if irritation persists.
Note to Physician:
Gastric lavage with 3 - 5% sodium bicarb to decrease absorption of salicylate ion.
Fire Fighting Measures
Fire:
As with most organic solids, fire is possible at elevated temperatures or by contact
with an ignition source.
Explosion:
Fine dust dispersed in air in sufficient concentrations, and in the presence of an
ignition source is a potential dust explosion hazard.
Fire Extinguishing Media:
Water spray, dry chemical, alcohol foam, or carbon dioxide.
Special Information:
In the event of a fire, wear full protective clothing and NIOSH-approved selfcontained breathing apparatus with full facepiece operated in the pressure demand
or other positive pressure mode.
Accidental Release Measures
Ventilate area of leak or spill. Wear appropriate personal protective equipment as
specified in Section 8. Spills: Sweep up and containerize for reclamation or
disposal. Vacuuming or wet sweeping may be used to avoid dust dispersal. Small
amounts of residue may be flushed to sewer with plenty of water.
Handling and Storage
Keep in a tightly closed container. Protect from physical damage. Store in a cool,
dry, ventilated area away from sources of heat, moisture and incompatibilities.
Chapter 9
Cost Estimation
Store in the dark. Containers of this material may be hazardous when empty since
they retain product residues (dust, solids); observe all warnings and precautions
listed for the product.
Exposure Controls/Personal Protection
A system of local and/or general exhaust is recommended to keep employee
exposures as low as possible. Local exhaust ventilation is generally preferred
because it can control the emissions of the contaminant at its source, preventing
dispersion of it into the general work area. Please refer to the ACGIH document,
Industrial Ventilation, A Manual of Recommended Practices, most recent edition,
for details.
For conditions of use where exposure to dust or mist is apparent and engineering
controls are not feasible, a particulate respirator (or better filters) may be worn. If
oil particles (e.g. lubricants, cutting fluids, glycerine, etc.) are present, use a P
filter. For emergencies or instances where the exposure levels are not known, use
a full-face positive-pressure, air-supplied respirator. Air-purifying respirators do
not protect workers in oxygen-deficient atmospheres.
Wear protective gloves and clean body-covering clothing.
Use chemical safety goggles. Maintain eye wash fountain and quick-drench
facilities in work area.
Disposal Considerations
Whatever cannot be saved for recovery or recycling should be managed in
an appropriate and approved waste disposal facility. Processing, use or
contamination of this product may change the waste management options. State and
local disposal regulations may differ from federal disposal regulations. Dispose of
container and unused contents in accordance with federal, state and local
requirements.
Facilities storing or utilizing this material should be equipped with an eyewash
facility and a safety shower. Use adequate general or local exhaust ventilation to
Chapter 9
Cost Estimation
keep airborne concentrations below the permissible exposure
Personal
Protective
Equipment
Wear appropriate protective eyeglasses or chemical safety goggles
limits.
Wear appropriate protective gloves to prevent skin exposure.
Wear appropriate protective clothing to prevent skin exposure.
A respiratory protection program followed whenever workplace conditions
warrant a respirator's use.
Other Information
NFPA
Ratings:
Label
Health:
1
Flammability:
Hazard
1
Reactivity:
0
Warning:
WARNING! HARMFUL IF SWALLOWED. AFFECTS CENTRAL NERVOUS
SYSTEM. MAY CAUSE IRRITATION TO SKIN, EYES, AND RESPIRATORY
TRACT
Avoid
Label
contact
Wash
with
Precautions:
eyes,
skin
thoroughly
Avoid
and
after
handling.
breathing
Keep
dust.
container
Use
with
Label
clothing.
closed.
adequate
ventilation.
First
Aid:
If swallowed, induce vomiting immediately as directed by medical personnel.
Never give anything by mouth to an unconscious person. Get medical attention. In
case of contact, immediately flush eyes or skin with plenty of water for at least 15
minutes. Get medical attention if irritation develops or persists. If inhaled, remove
to
fresh
air.
Get
medical
attention
for
any
breathing
difficulty.
Chapter 9
Cost Estimation
Chapter No.9
Cost Estimation
Chapter No.9
Cost Estimation
Chapter 9
Cost Estimation
Equipment
Number
Capacity
Autoclave
3
CFSTR
1
10.332
m3
1.86 m 3
Acidification
tank
Centrifuge
3
4.2 m3
……..
3
7.44 m3
………
Dryer
1
4.95m2
30000
30000
………
Distillation
Column
Flash Tank
1
10 m
25000
25000
………
1
19.5 m3
14000
14000
………..
10500
21000
………..
9000
9000
……….
2
Cost/equipment
Total cost
Reference:
Peter and
Timmerhause
………
Condenser
2
100 m
Reboiler
1
80 m2
Storage tank
(NaOH)
Storage Tank
(H2SO4)
Storage Tank
(CO2)
Mixing Tank
1
…………
1
………..
1
………..
2
………..
Storage
tank(Phenol)
Pumps
1
…………
8
…………
Compressor
1
………..
Sublimation
Tank
Crystallizer
1
………. ..
1
………..
Direct Cost
Purchased equipment cost = E =
Installation cost ( 40%E) =
Instrumentation and control cost (13%E) =
Chapter 9
Cost Estimation
Piping cost
(10% E)
Electric cost
(10%E) =
=
Building (including services) (29%E =
Yard improvement (10%E) =
Land cost (6%E)
=
Total Direct Cost
Indirect cost
Engineering and Supervision Cost (32%E) =
Constriction Expenses (30%E)
Contractor fee (18%E)
=
=
Contingency Cost (30%E) =
Total Indirect Cost
Fixed Capital Investment = Direct cost + Indirect cost
F.C.I
= D.C
+
I.C
Working Capital (18%F.C.I) =
Total Capital Investment
T.C.I
= Working Capital Cost +Fixed Capital Investment
= W.C
+
F.C.I
Product Cost
Assume that the Fixed Capital Investment depreciate by straight line method for
20 years. Assuming 5 % Salvage value at the end of plant life.
Depreciation = D = (V-VS)/N
V = F.C.I
VS = 0.05*F.C.I
N =no. of years =20
D=
Total Product Cost = Total Capital Investment – Depreciation
=
Chapter 9
Cost Estimation
Fixed Charges (12%T.P.C) =
Direct Product Cost (55%T.P.C) =
Plant Overhead (10%T.P.C) =
General Expenses
=
Plant Capacity = 25*330 =8250 ton per year (year of 330 days)
Selling Price =
Total income = Selling Price *production =
Total Profit before Taxes = Income – Expenses =
Return on Investment = Total Profit
Plant overhead
Chapter 9
Cost Estimation
REFERENCES
1)
Ludwig, E.E, “Applied Process Design”, 3rd ed, vol.
2, Gulf Professional Publishers, 2002.
2)
Ludwig, E.E, “Applied Process Design”, 3rd ed, vol.
3, Gulf Professional Publishers, 2002.
3)
McKetta, J. J., “Encyclopedia of Chemical Processing
and Design”, Executive ed, vol. 1, Marcel Dekker Inc, New York, 1976.
4)
Kuppan, T., “Heat Exchanger Design Hand Book,
Marcel Dekker Inc., New York, 2000.
5)
Levenspiel, O., “Chemical Reaction Engineering:, 2nd
ed, John Wiley and Sons Inc., 1972.
6)
Peters, M.S. and Timmerhaus, K.D., “Plant Design
and Economics for Chemical Engineering”, Fourth ed, McGraw Hill,
1991.
7)
Bockhurst, J.F. and Harker, J.H, “Process Plant
Design”, Heinemann Educational Books Ltd, 1973.
8)
Coulson, J.m., and Richardson, J.F., “Chemical
Engineering”, 4th ed, Vol.2, Butterworth Heminann, 1991.
9)
Peacock, D.G., “Coulson & Richardson’s Chemical
Engineering”, 3rd ed, vol, Butterworth Heinenann, 1994.
Chapter 9
10)
Cost Estimation
Sinnot, R.K., “Coulson and Richardson’s Chemical
Engineering”, 2nd ed, vol 6, Butterword Heinemann, 1993.
11)
Kern, D.Q., “Process Heat Transfer”, McGraw Hill
Inc., 2000.
12)
McCabe, W.L, “Unit Operations of Chemical
Engineering”, 5th Ed, McGraw Hill, Inc, 1993.
13)
Perry, R.H and D.W. Green (eds): Perry’s
Chemical Engineering Handbook, 7th edition, McGraw Hill
New York, 1997.
14)
Holland,H.A.and
Chapman,F.S.,”Liquid
Mixing and Procesing in Stirred Tanks”,Liver Brother
Company, New York.
15)
Harnby,H.,Edward,M.F.
Nienow,A.W.,”Mixing
in
and
the
Process
Industries”,ButterWorths ,London,
16)
www.nist.com
17)
www.uspto.gov
18)
www.haverstandard.com
Do
D i
Chapter 9
Cost Estimation