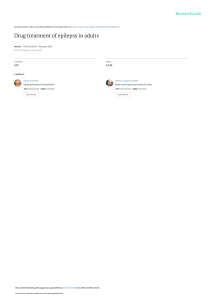
The neuroendocrine basis of sex differences in epilepsy Doodipala Samba Reddy PII: DOI: Reference: S0091-3057(16)30121-6 doi: 10.1016/j.pbb.2016.07.002 PBB 72381 To appear in: Pharmacology, Biochemistry and Behavior Received date: Revised date: Accepted date: 25 March 2016 25 June 2016 12 July 2016 Please cite this article as: Reddy Doodipala Samba, The neuroendocrine basis of sex differences in epilepsy, Pharmacology, Biochemistry and Behavior (2016), doi: 10.1016/j.pbb.2016.07.002 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. ACCEPTED MANUSCRIPT Revised Manuscript # PBB-D-16-00125 Invited Review Pharmacology, Biochemistry and Behavior (Special Issues: Behavioral Sex Differences; Editor: Dr. Alonso Fernández-Guasti) PT The Neuroendocrine Basis of Sex Differences in Epilepsy Doodipala Samba Reddy SC RI Department of Neuroscience and Experimental Therapeutics, College of Medicine, Texas A&M University Health Science Center, Bryan, TX 77807, USA MA Running title: Sex differences in epilepsy NU Correspondence: [email protected] (Prof. D. S. Reddy) Manuscript: Total text pages = 18 (total words = 5,330) TE D Abstract = 140 words; References = 132 ------------------------------------------------------------------------------------------------------------------------------------- AC CE P ABSTRACT Epilepsy affects people of all ages and both genders. Sex differences are well known in epilepsy. Seizure susceptibility and the incidence of epilepsy are generally higher in men than women. In addition, there are gender-specific epilepsies such as catamenial epilepsy, a neuroendocrine condition in which seizures are most often clustered around the perimenstrual or periovulatory period in adult women with epilepsy. Changes in seizure sensitivity are also evident at puberty, pregnancy, and menopause. Sex differences in seizure susceptibility and resistance to antiseizure drugs can be studied in experimental models. An improved understanding of the neuroendocrine basis of sex differences or resistance to protective drugs is essential to develop targeted therapies for sex-specific seizure conditions. This article provides a brief overview of the current status of sex differences in seizure susceptibility and the potential mechanisms underlying the gender differences in seizure sensitivity. Keywords: Catamenial epilepsy; Epileptogenesis; Sex difference; Seizure; Pilocarpine; Neurosteroid -------------------------------------------------------------------------------------------------------------------------- 1. Introduction Epilepsy has many causes. Sex differences are well known in epilepsy, which is characterized by an enduring predisposition to recurrent seizures. A seizure is an abnormal electrical storm in the brain that causes sudden alterations in consciousness, sensation and behavior that can manifest in forms ranging from an eye flicker to full-body convulsions. Epileptic seizures arise from dysfunctional neuronal network mechanisms that regulate excitability and synchrony. Epileptic seizures are classified into partial (simple partial and complex 1 ACCEPTED MANUSCRIPT partial) and generalized (absence, tonic-clonic, myoclonic, and atonic seizures) types. Every year, nearly 150,000 new cases of epilepsy are diagnosed in the United States (Hesdorffer et al., 2013). Despite the availability of many medications, nearly 30% of people with epilepsy have refractory seizures that do not respond to any of the currently available treatment options. MA NU SC RI PT Catamenial epilepsy is a type of gender-specific epilepsy in which seizures are clustered around a particular phase of the menstrual cycle. This condition affects as many as 70% of women with epilepsy (Reddy, 2003; 2016a). Most of these patients suffer from uncontrollable seizures, which could damage the brain and adversely impact their quality of life. Therefore, there is a large gap in our understanding of sex differences in epileptic seizures and symptomatic antiepileptic medications’ control of a disease with no cure. Epilepsy can be either caused by certain genetic defects or acquired from a predisposing brain injury. Consequently, there are several experimental models that capture a few of these features. Sex differences are also evident in experimental models of seizure susceptibility and epileptogenesis, which occurs following a precipitating insult or injury, such as traumatic brain injury, stroke, neurotoxicity, brain infections, or prolonged seizures (Reddy, 2009a; 2013a,b). In 2014, the NIH issued a policy about the inclusion of both genders in the preclinical research (Clayton and Collins, 2014). This article provides a brief overview of the current status of sex differences in epilepsy and the potential mechanisms underlying the sex differences in seizure sensitivity. D 2. Sex differences in clinical epilepsy AC CE P TE The issue of sex differences in seizure susceptibility has been long-standing in the study of epilepsy. Clinical evidence shows gender- and age-related expression in many seizure syndromes. The incidence of epilepsy is generally higher in males than in females; however, the prevalence depends a lot on the specific form of epilepsy (Hauser, 1997; Christensen et al., 2005). More women than men are diagnosed with idiopathic generalized and cyptogenic localization-related epilepsies, but localization-related symptomatic epilepsies are more frequent in men (Hauser, 1997; Christensen et al., 2005). In general, men are more susceptible to injuryinduced seizures than women. Additionally, there is a higher frequency of infantile spasms, an age-specific epileptic syndrome affecting infants and young children, in boys than girls. Furthermore, some findings have shown that in early-onset temporal lobe epilepsy, women show greater functional plasticity for verbal memory than men. The relationship between menstrual cycle and seizure sensitivity in women is well known and is greatly influenced by hormonal fluctuations associated with menstrual cycle phases. However, a recent review yielded no consistent evidence of gender differences in the incidence or consequences of these epilepsies (Perucca et al., 2014). Nevertheless, there is considerable evidence indicating that males exhibit greater seizure susceptibility, while many females exhibit greater fluctuations in susceptibility to seizures, including menstrual cycle-related changes in seizure activity. Growing literature also suggests that the incidence of epilepsy differs between men and women (Savic and Angel, 2014). In most countries, not only is the incidence of epilepsy lower in women than men, but it has also been reported that males have a higher lifetime risk of developing epilepsy (Sridharan and Murthy, 1999; McHugh and Delanty, 2008; Benamer and Grosset, 2009; Hesdorffer et al., 2011; Kim et al., 2014), though there are some inconsistencies across studies due to a number of factors (Scharfman and MacLusky, 2014). When specific subtypes of epilepsy are selectively studied, there are more compelling gender differences (Christensen et al., 2005). For example, idiopathic generalized epilepsy is more common in women (McHugh and Delanty, 2008), as is a type of reflex epilepsy called photosensitive epilepsy (Taylor et al., 2007). Alternatively, focal cortical dysplasia is more common in males (Ortiz-Gonzalez et al., 2013), and males have a higher prevalence for a different type of malformation, perinodular heterotopia (Sisodiya et al., 1999). 2 ACCEPTED MANUSCRIPT NU SC RI PT A detailed review discussing the gender differences in temporal lobe epilepsy (TLE) has been published recently (Koppel and Harden, 2014). TLE is characterized by a progressive expansion of spontaneous seizures stemming from the limbic system regions, especially the hippocampus, and is often drug resistant. In essence, several aspects of TLE appear to differ in men and women. These aspects include auras, which are more common in females (Janszky et al., 2004), as well as differences in lateralization and generalization of seizures (Janszky et al., 2004). Voxel based morphometry shows abnormalities in men with TLE that are frontal, whereas, in women they are often more temporal (Santana et al., 2014). Reduced metabolism within the extratemporal region has been found to be more common in men than women with TLE (Savic and Engel, 1998; Nickel et al., 2003). Male preference is also reported in special epilepsy syndromes like Landau–Kleffner syndrome, epilepsy with continuous spike and wave complexes in slow wave sleep, epilepsy with myoclonic absences, West or Dravet syndromes, and benign epilepsy with centrotemporal spikes (Panayiotopoulos, 2007). Conversely, female preponderance was reported in juvenile myoclonic epilepsy (Camfield and Camfield, 2009; Janz, 1998), childhood absence epilepsy, perioral myoclonic with absences, and myoclonic encephalopathy in non-progressive disorders (Panayiotopoulos, 2007; van Luijtelaar et al., 2014). TE D MA Seizures do not occur randomly and tend to cluster in the majority of men and women with epilepsy; however, there are some sex-specific forms of epilepsy. Many of these are based on conditions that are mostly genetically-determined or based on natural fluctuations in hormonal status. Given the recent advances in genetic technology and genetic testing, epileptologists are now able to diagnose such patients more easily. However, there are many questions about the diagnosis and management of these genetic epilepsies, such as: When should these diagnoses be thought of? How are the seizures in these conditions? What other neurological, psychiatric and systemic problems are associated? What is the best treatment? These issues related to genetically determined epilepsies in both men and women will be addressed in future research. AC CE P There is little information on the clinical characteristics of genetically determined epilepsy that only affects women. PCDH19 is a serious and rare epileptic syndrome that affects only pediatric female patients— approximately 15,000-30,000 females in the United States (Tan et al., 2015; Ikeda et al., 2016). The condition, which is caused by an inherited mutation of the protocadherin 19 (PCDH19) gene, located on the X chromosome, is characterized by early-onset cluster seizures, cognitive and sensory impairment of varying degrees, and behavioral disturbances. The PCDH19 gene encodes a protein, protocadherin 19, which is part of a family of molecules supporting the communication between cells in the central nervous system. In case of mutation, protocadherin 19 may be malformed, reduced in its functionality or not produced at all. The abnormal expression of protocadherin 19 is associated with the occurrence of seizures beginning in the early years of life, mostly consistent of focal clustered seizures that last from one day to weeks. Often, but not always, the syndrome is also associated with a cognitive impairment of varying nature, and behavioral or social disorders with autistic traits. Currently, there are no approved therapies for PCDH19 female pediatric epilepsy. Neurosteroids, such as the synthetic GABA-A receptor-modulating ganaxolone, are proposed as symptomatic treatments in female children with epilepsy caused by a mutation of the PCDH19 gene. This epilepsy is characterized by cluster seizures and behavioral disturbances in girls. It is thought that the uncontrolled seizures are linked to PCDH19 mutation and to low levels of allopregnanolone, a naturally occurring neurosteroid in the brain (Lotte et al., 2015). Many women with epilepsy experience a type of refractory epilepsy known as catamenial epilepsy; seizures exacerbate with menstrual fluctuation of sex hormones (Reddy, 2016a). The periodicities may differ between women with ovulatory and anovulatory cycles. There is emerging information on the role of sex hormones in pathogenesis of seizure exacerbation in catamenial epilepsy and whether the response to treatment can be predicted. The neuroactive properties of reproductive steroids and the variation of their serum 3 ACCEPTED MANUSCRIPT RI PT concentrations in relation to the phases of the menstrual cycle may be critical factors for the development of catamenial seizure exacerbation. There is also some evidence to suggest that the laterality and focality of epilepsy may be a factor in the level of susceptibility to these presumed hormonal influences. Three types of catamenial seizures have been identified: perimenstrual (C1), periovulatory (C2), and inadequate luteal-phase (C3) (Herzog et al., 1997). Perimenstrual catamenial epilepsy is the most common clinical type. In perimenstrual catamenial epilepsy (C1), women with epilepsy experience an increase in seizure activity before, during, or after the onset of menstruation (Reddy, 2009a). The diagnosis of ovulatory or anovulatory cycles is often made by estimating the midluteal phase progesterone levels. Progesterone levels lower than 5 ng/ml during days 20 through 22 of the cycle would certainly indicate an inadequate luteal phase. AC CE P TE D MA NU SC It is essential to discuss the effects of antiepileptic drugs and seizures on female reproductive function from puberty to menopause, as well as open a conversation about the current knowledge of the complex interactions between seizures, sex hormones, brain physiology, and medications in order to implement meaningful treatment approaches for women with epilepsy. Catamenial epilepsy is a multifaceted condition attributed to numerous causes. Catamenial epilepsy is an acquired disorder and currently there is no clear evidence of genetic components (Herzog, 2009; Quigg et al., 2009). A variety of mechanisms including fluctuations in antiepileptic drug levels, changes in water and electrolyte balance, and physiological variation in ovarian hormone secretion have been proposed as causes for catamenial epilepsy (Reddy et al., 2001; Gilad et al., 2008; Reddy, 2013a; Reddy, 2013b). Estradiol has been known to play a role in the exacerbation of seizures in women with epilepsy (Logothetis et al., 1959; Backstrom, 1976; Jacono and Robinson, 1987; Younus and Reddy, 2016). Plasma estradiol levels are found to increase during both the follicular and luteal phase of the normal menstrual cycle. Thus, an increase in the ratio of estrogen-to-progesterone levels during the perimenstrual period might at least partly contribute to the development of perimenstrual seizure exacerbation (Bonuccelli et al., 1989; Herzog et al., 1991). Progesterone plays a key role in catamenial epilepsy. Progesterone has long been known to have antiseizure activity in a variety of animal models of epilepsy (Craig, 1966; Backstrom et al., 1984; Landgren et al., 1978; Reddy, 2009a). Progesterone also has antiepileptogenic actions. The antiseizure actions of progesterone are mostly mediated by its metabolic conversion into neurosteroids (Reddy, 2004a; 2004b; Reddy, 2010; Reddy and Mohan, 2011; Reddy, 2011; Reddy and Ramanathan, 2012). Changes in progesterone levels have been directly correlated with catamenial seizures (Reddy et al., 2004; Tuveri et al., 2008; El-Khayat et al., 2008). An extrasynaptic molecular mechanism involving tonic inhibition is shown to play a critical role in catamenial seizures and drug sensitivity (Reddy, 2016a). 3. Sex differences in experimental seizures Sex differences in seizure susceptibility are well recognized in preclinical models. In animals, acute seizures can be modeled in several ways, usually involving electrical stimulation or chemical stimulation by means of a chemoconvulsant. The maximal electroshock (MES), which models generalized tonic-clonic seizures, and acute pentylenetetrazol (PTZ) injection, which models clonic seizures, are widely used to discover antiepileptic drugs and determine the seizure susceptibility or threshold following pharmacological interventions. Seizure susceptibility is determined by measuring the animal’s response to chemoconvulsants such as PTZ or other GABAergic antagonists (such as bicuculline or picrotoxin). Kindling is a widely used model for epileptogenesis and seizure expression, and has been used to model complex partial seizures. Chronic epilepsy models with spontaneous seizures are less frequently used but provide several criteria or phenotypes of a human epileptic condition. In acquired epilepsies, spontaneous seizures begin after injury to a normal brain as a consequence of trauma, stroke, infection or status epilepticus (Staley, 2015). Such chronic 4 ACCEPTED MANUSCRIPT epilepsy is modeled using pilocarpine or kainic acid which can induce status epilepticus (SE), a state of continuous seizures that lasts for hours before self-terminating. SE in the rodent often initiates a pattern of brain damage and other changes in the brain that trigger epileptogenesis, leading to spontaneous seizures and epilepsy within days or weeks (Reddy and Kuruba, 2013). AC CE P TE D MA NU SC RI PT Animal models are important tools for studying the hormonal aspects of epilepsy. Many experimental studies over the last 20 years have identified sex and cycle-dependent differences in seizure sensitivity in rats and mice (Genazzani et al., 1988; Schwartz-Giblin et al., 1989; Finn and Gee, 19994; Pericic et al., 1996; Bujas et al., 1997; Pericic and Bujas, 1997; Reddy and Rogawski, 2001; Tan and Tan, 2001; Valente et al., 2002; Scharfman et al., 2005; Reddy and Rogawski, 2009). In most animal models of induced seizures, male mice or rats are more seizure-prone than females. For example, our experimental results show male adult mice to have a lower seizure threshold than females in the pentylenetetrazol test (Reddy, 2009b). Sex differences in the occurrence or severity of seizures are found for both GABAergic and glutamatergic compounds. The most marked differences are observed with ovariectomies, gonadectomies, and pharmacological manipulations of reproductive milieu. In experimental animals, estrous cycle related changes in seizure susceptibility are widely reported in literature (Wu et al., 2013; Scharfman and MacLusky, 2014). Mice exhibit a 6-day ovarian/estrous cycle, which is subdivided into four stages (estrus, diestrus, metestrus and estrus) that are associated with distinct hormonal milieu. Estrous cycle stage can be determined by microscopic examination of vaginal smears with eosin staining (Wu et al., 2013). Diestrus is characterized by high progesterone relative to the estrus stage. As is seen clinically, the hormonal milieu present during the diestrus phase reduces seizure susceptibility, but animals are more vulnerable to seizures during the proestrus or estrous phase. Progesterone levels are significantly higher in diestrus compared with levels during estrus. In contrast, the chronic absence of an estrous cycle, such as that induced by ovariectomy, leads to a greater chronic susceptibility to seizures. Female rats were found to have a significantly higher susceptibility to the organophosphate agent sarin when in the proestrus stage of their cycle when compared to age-matched counterparts that were in estrous or had been ovariectomized (Smith et al., 2015). This study has translational implications for nerve agent seizures (Reddy, 2016b). Sex differences in animals show that the most robust differences are only at certain ages, at certain stages of the female ovarian cycle, and vary across different brain areas; therefore, it is necessary to account for all stages of the estrous cycle when conducting experiments on female rodents. Sex differences are evident with pilocarpine and kainic acid-induced SE. There is a growing consensus in favor of a striking resistance of female rats to pilocarpine or lithium-pilocarpine compared to males (Persinger et al., 1988; Scharfman et al., 2005; Scharfman and MacLusky, 2014). The results are consistent with the relative resistance of female mice to pilocarpine-SE (Buckmaster PS and Haney, 2012). Males were more likely to develop SE than females, but sexes were equally likely to survive status epilepticus. Consequently, male mice were 1.3-times more likely to develop SE and survive than females after pilocarpine administration. The resistance of females to cholinergic drugs that influence seizures in males was also shown in DBA/2J mice with respect to audiogenic seizures (Lonsdale, 1982). Previous studies had suggested that there was no such evidence for sex differences in SE using kainic acid acute seizures (Scharfman and MacLusky, 2014); however, new findings suggest that males are more susceptible to kainic acid induced TLE with greater spontaneous recurrent seizures (Twele et al., 2016). Paradoxically, there was a shorter latency period in female mice than in male mice. Furthermore, the age of the mice can have a key effect on sensitivity to kainic acid; aged female mice showed greater neurodegeneration when compared to aged male mice and to adult mice of both sexes (Zhang et al., 2008). These divergent results suggest differences in receptor mechanisms rather than general excitability that account for sex differences in SE susceptibility. Moreover, in some animal models of absence epilepsy, females exhibit predominance in spike and wave rhythmic seizures (Persad et al., 2002; Li et al., 2007). In another study, female periadolescent rats develop nicotine-kindled seizures earlier than their male counterparts (Gomes et al., 2013). Oxidative stress has been implicated in the pathophysiology of seizures and is also related to seizure-induced 5 ACCEPTED MANUSCRIPT PT neurodegeneration. Differences in the oxidative balance or related inflammation may be involved in this mechanism (Reddy et al., 2016). Neuroinflammation is a common consequence of seizures and related neuronal injury events. Brain inflammation is emerging to play a central role in the pathogenesis of acquired epilepsy (Vezzani et al., 2013; Rojas et al., 2014). There are many components of neuroinflammation that are linked to sex differences including hormones, immunological changes, and neuronal network organization. Therefore, sex differences in such factors that control inflammation could likely contribute to sex based differences in the incidence of epilepsy. MA NU SC RI In contrast to acute seizures, sex differences in epileptogenesis or acquired epilepsy is more complex due to variations in hormonal factors. There is little literary evidence of a direct comparison of the rates of epileptogenesis in males and females. Even when all variables are controlled, there are unexpected endocrine defects that confound the outcomes. In kindling, for example, estrous cycles of female rats become abnormal (Edwards et al., 1999). This is also common in animal models of TLE that use SE as the initial insult (Amado and Cavalheiro, 1998; Scharfman et al., 2009). Polycystic ovaries and increased androgen levels develop (Scharfman et al., 2008). Consequently, it is difficult to discern sex differences in epileptogenesis because inducing convulsive seizures changes the reproductive endocrinology of the female rodent. AC CE P TE D Increasing evidence shows that gender and age is an important factor in many neurological disease states, including epilepsy. Some studies have reported that there are no gender differences in seizure susceptibility in rat pups; however, normal adult male rats are more susceptible to seizures induced by pilocarpine, kainic acid, MES, hyperthermia, and PTZ. However, it is important to note that female rats that had experienced febrile seizures as pups are significantly more prone to developing epilepsy in adulthood than males that had experienced febrile seizures and to age-matched normal rats of both sexes (Dai et al., 2014). This data suggests a sex-dependent phenomenon of acquired seizure susceptibility after complex febrile seizures. 4. Neuroendocrine mechanisms underlying sex differences in seizures Sex-based differences in seizure sensitivity may arise from variations between men and women in factors such as body weight, steroid hormones, cytochrome P450 activity, neurotransmitter systems, and biological differences in neuronal networks in the brain (Cooke et al., 1999; Veliskova and Moshe, 2001; Ravizza et al., 2003; Reddy, 2009b) (Figure 1). Steroid hormones play a key role in the neuroendocrine control of neuronal excitability and seizure susceptibility in men and women with epilepsy (Herzog, 1991; Verrotti et al., 2007; Reddy, 2010a; 2013a; 2014). Changes in seizure sensitivity are also evident at puberty, which is associated with rigorous changes in reproductive hormones and behavioural patterns (Reddy, 2009a). The relationship between the menstrual cycle and seizure sensitivity in females is well known and is greatly influenced by hormonal fluctuations associated with menstrual cycle phases (Bazan et al., 2005). There is growing awareness that a key modulatory system in the brain, the endocannabinoid system, may differ between males and females (Wiley et al., 2008; Reich et al., 2009; Atkinson et al., 2010). Endocannabinoids are involved in diverse aspects of physiology and behavior that involve the hippocampus, including cognitive and motivational states, responses to stress, and neurological disorders such as epilepsy. A recent finding that molecular regulation of the endocannabinoid system differs between the sexes is suggestive of mechanisms through which experiences or therapeutics that engage endocannabinoids could affect males and females differently (Tabatadze et al., 2015). Steroid hormones are involved in sex differences in epilepsy (Figure 1). Progesterone, estrogen and androgen are known to affect seizure susceptibility (Reddy, 2010). Progesterone is an anticonvulsant hormone. Estrogen is both a proconvulsant and anticonvulsant depending on the physiological status. Androgens are 6 ACCEPTED MANUSCRIPT NU SC RI PT bimodal modulators of seizure susceptibility (Reddy and Zeng, 2007). Unlike progesterone, the potential pathways for the testosterone modulation of seizure activity are complex. Testosterone is known to produce both proconvulsant and anticonvulsant effects depending on the animal model and the seizure type (Werboff and Havlena, 1968; Thomas and McLean, 1991; Frye and Reed, 1998; Pesce et al., 2000; Mejias-Aponte et al., 2002). Both animal and clinical studies show that testosterone enhances seizure activity by metabolism to estrogens (see Reddy, 2008). Epidemiological data indicates that the occurrence of focal and tonic–clonic epileptic seizures is 50% higher in intact than in castrated dogs (VMDB Report, 2003). More detailed reviews of the hormonal effects on seizures and gender differences in epilepsy were published previously (Reddy, 2014; Scharfman and MacLusky, 2014). The differing levels of these various steroid hormones between males and females draw an obvious conclusion that endocrinology has an effect on seizure susceptibility, and therefore, epilepsy. In turn, this begins to explain some of the differences in prevalence that we see between males and females because of elevated levels of circulating neuroprotective hormones such as progesterone and estrogen when comparing males and females of similar age. AC CE P TE D MA Neurosteroids play a critical role in sex differences in seizure susceptibility. Neurosteroids are steroids synthesized within the brain and which can rapidly alter neuronal excitability. Steroid hormones such as progesterone and deoxycorticosterone can exert anticonvulsant actions. Experimental studies have demonstrated that the anticonvulsant properties of progesterone and deoxycorticosterone are due to their conversion to the neurosteroids allopregnanolone (3-hydroxy-5-pregnane-20-one) and allotetrahydrodeoxycorticosterone (3,21-dihydroxy-5-pregnan-20-one; THDOC), respectively (Reddy, 2010). A variety of neurosteroids are known to be synthesized in the brain, the most widely studied being allopregnanolone, THDOC, and androstanediol. These are produced via sequential A-ring reduction of the steroid hormones by 5-reductase and 3-hydroxysteroid oxidorectase isoenzymes (Reddy, 2009a). The androgenic neurosteroid androstanediol (5-androstan-3,17β-diol) is synthesized from testosterone (Reddy, 2004a; 2004b). In the periphery, the steroid precursors are mainly synthesized in the gonads, adrenal gland, and feto-placental unit, but synthesis of these neurosteroids likely occurs in the brain from cholesterol or from peripherally derived intermediates. Since neurosteroids are highly lipophilic and can readily cross the bloodbrain barrier, neurosteroids synthesized in peripheral tissues accumulate in the brain. Neurosteroids modulate neuronal excitability through direct interaction with GABA-A receptors (Hosie et al., 2007; Saalmann et al., 2007). Allopregnanolone and other similar neurosteroids act as positive allosteric modulators and direct activators of GABA-A receptors (Figure 1). Consequently, neurosteroids are often referred to as endogenous modulators of GABA-A receptors in the brain (Carver and Reddy, 2013; 2014; 2016; Reddy and Estes, 2016). GABA-A receptors are responsible for the majority of inhibitory currents in the brain. Structurally, these receptors are pentameric channels made from various subunits (α1-6, β1-4, γ1-3, δ, ε, θ, ρ1-3). GABA-A receptors are ligand-gated chloride channels which, when activated by GABA, hyperpolarize the neurons through influx of chloride ions. Based on location, GABA-A receptors are categorized into synaptic and extrasynaptic receptors. Synaptic (γ-containing) receptors, which are present ubiquitously within the brain, produce phasic currents in response to the vesicular release of GABA. Extrasynaptic (δ-containing) receptors, which are expressed in specific brain regions including the hippocampus, thalamus, amygdala, and cerebellum, generate non-desensitizing tonic currents that are continuously gated by extracellular GABA. Neurosteroids are potent positive allosteric agonists of synaptic and extrasynaptic GABA-A receptors (Reddy and Estes, 2016). The mode of action for neurosteroids depends on the concentration. At low concentrations, neurosteroids potentiate GABA-A receptor currents, whereas at higher concentrations, they directly activate 7 ACCEPTED MANUSCRIPT AC CE P TE D MA NU SC RI PT the receptor (Harrison et al., 1987; Reddy and Rogawski, 2002). Like barbiturates, neurosteroid enhancement of GABA-A receptors occurs through increases in both the channel open frequency and channel open duration. The effect of neurosteroids on GABA-A receptors occurs by binding to discrete sites on the receptor-channel complex that are located within the transmembrane domains of the - and -subunits (Hosie et al., 2006; Carver and Reddy, 2013), and that they access these sites by lateral membrane diffusion (Chisari et al., 2010). The binding sites for neurosteroids are distinct from the recognition sites for GABA, benzodiazepines, and barbiturates (Hosie et al., 2009). Androgenic neurosteroids such as androstanediol may interact with these sites and our study indicates that this agent is a positive allosteric modulator of GABA-A receptors (Reddy and Jian, 2010). Although neurosteroids act on all GABA-A-receptor isoforms, they cause large effects on extrasynaptic δ-subunit containing isoforms that mediate tonic currents (Wohlfarth et al., 2002; Belelli et al., 2002). Neurosteroids therefore could play a role in setting the level of excitability by potentiation of tonic inhibition during seizures when ambient GABA may rise. Like other GABAergic agents, neurosteroids are powerful anticonvulsants (Reddy, 2010). Natural and synthetic neurosteroids exhibit broad-spectrum anticonvulsant effects in diverse rodent seizure models (Reddy, 2003; Reddy and Woodward, 2004; Reddy and Rogawski, 2010). They protect against seizures induced by GABA-A receptor antagonists, including pentylenetetrazol and bicuculline, and are effective against pilocarpineinduced limbic seizures, 6-Hz stimulation induced limbic-like seizures, and seizures in kindled animals (Reddy et al., 2010). Neurosteroids can effectively control organophosphate-intoxication induced seizures (Reddy, 2016b). There is emerging evidence that endogenous neurosteroids play a role in sex differences in seizure susceptibility and drug response (Pericic et al., 1986; Reddy et al., 2004; Biagini et al., 2006; Reddy, 2009b). Although there is no evidence that alterations in neurosteroid levels in the absence of preexisting epilepsy can induce epileptogenesis, it is likely that alterations in neurosteroid synthesis may influence occurrence of seizures. Our preclinical work provides important new evidence that the availability of neurosteroids does indeed critically influence the propensity for seizures (Reddy and Zeng, 2007). We used epileptic female rats that had experienced status epilepticus. Spontaneous seizure activity was monitored for up to 5 months. The epileptic animals exhibited about 2 seizures per day, each lasting approximately a minute. Gonadotropin induced increase in neurosteroids was associated with reduced seizure intensity. However, when neurosteroids were withdrawn, using the neurosteroid synthesis inhibitor finasteride, a significant (two-fold) increase in seizure frequency was observed (Reddy, 2009a). These findings are confirmed in an independent study by Lawrence et al (2010) using ovariectomized epileptic animals. Since endocrine fluctuations in plasma levels of progesterone and other steroids can mediate neurosteroid availability, there are apparent differences between males and females concerning concentrations of neurosteroids in the brain. In addition, brain development greatly differs between genders and likely contributes to neurosteroid function (Reddy, 2009b). While neurosteroids are able to shape inhibition and produce behavioral effects in both genders, regulation of neurosteroid activity may be sex-specific (Gulinello and Smith, 2003). Differences in maximal GABAA receptor potentiation are observed between male and female rats for THDOC, but not for allopregnanolone or androgenic neurosteroids (Wilson and Biscardi, 1997). Gender differences in expression of 3α-hydroxysteroid dehydrogenase are evident during puberty, but these differences subside in the brain as it matures into adulthood; sex-specific gonadal and adrenal endocrine activity have a significant effect on the ability of allopregnanolone to modify anxiolytic actions based on variations in biosynthesis of steroid hormones (Mitev et al., 2003). Sex differences are evident in the anticonvulsant activity of neurosteroids; however, the potential mechanisms remain unclear. It is likely that differences in post-synaptic or extrasynaptic GABA-A receptor expression and function may underlie the sex differences in seizure sensitivity and the anticonvulsant activity of neurosteroids. 8 ACCEPTED MANUSCRIPT SC RI PT When neurosteroid levels fluctuate, loss of seizure control can occur. A key clinical situation in which neurosteroids are a factor in seizure control is catamenial epilepsy (Reddy and Rogawski, 2000ab; Reddy, 2009a). Neurosteroids have been implicated in perimenstrual seizure exacerbations in women with normal menstrual cycle. It is hypothesized that withdrawal of progesterone-derived neurosteroids leads to enhanced excitability predisposing to seizures. In addition, plasticity in GABA-A receptor subunits could play a role in the enhanced seizure susceptibility in perimenstrual catamenial epilepsy. Animal studies have shown that prolonged exposure to allopregnanolone followed by withdrawal such as that occurs during menstruation causes a marked increase in expression of 4-subunit, a key subunit linked to enhanced neuronal excitability, seizure susceptibility and benzodiazepine resistance (Smith et al., 2007; Gangisetty and Reddy, 2010). Although 4 can coassemble with 2 to form synaptic GABA-A receptors, it preferentially co-assembles with to form extrasynaptic GABA-A receptors. Overall, these neuroendocrine changes can result in reduced inhibition resulting in enhanced excitability, which, among other effects, predisposes to seizures. NU 5. Conclusion AC CE P TE D MA Epilepsy may be the result of developmental problems due to genetic mutations that interfere with the normal wiring of the brain. It can also be caused by conditions such as infection, tumors, stroke, or any kind of injury to the brain. Sex differences in seizure susceptibility has been a long-standing issue in epilepsy. Clinical evidence shows gender- and age-related expression of many seizure syndromes. The incidence of epilepsy is generally higher in males than in females. More women than men are diagnosed with idiopathic generalized epilepsy, but localization-related symptomatic epilepsies are more frequent in men, and cryptogenic localization-related epilepsies are more frequent in women. Changes in seizure sensitivity are also evident at puberty, which is associated with rigorous changes in reproductive hormones and behavioural patterns. Despite some inconsistencies, there is considerable evidence indicating that men exhibit greater seizure susceptibility than women, while many women exhibit greater fluctuations in susceptibility to seizures, including menstrual cycle-related catamenial seizures. Although sex differences in epilepsy are widely recognized, there is little discussion on their mechanisms and therapeutic implications. Steroid hormones play a key role in gender differences in susceptibility to epileptic seizures. Neurosteroids are synthesized within the brain from circulating steroid hormones and they protect against seizures in males and females. There is a need for future epilepsy research to focus on the role of hormones, especially the class of neurosteroids, in the pathophysiology and treatment of epilepsy and its comorbidities, as well as the complex interactions between hormones and antiepileptic drugs that impact contraception, premenstrual syndrome, pregnancy and menopause. Neurosteroids, molecules generated in glia from circulating steroid hormones and de novo from cholesterol, keep seizures in check in epileptic animals. They can enhance inhibitory transmission mediated by GABA-A receptors and have an anticonvulsant action. Potential differences in circulating steroid hormones or neurosteroid levels in the brain in males and female may contribute to sex differences in seizure control. Consequently, differences in postsynaptic or extrasynaptic GABA-A receptor expression and function may account for the sex differences in seizure sensitivity and the anticonvulsant activity of neurosteroids. Thus, it is likely that endogenous neurosteroids are involved in gender differences in seizure susceptibility. Unfortunately, the endocrine system is not always taken into consideration when performing novel experimentation. It can become a financial burden to conduct studies on every stage of the estrous cycle in rodents, and for this reason it is more common to find studies only including male animal models. However, without these important data points it could be difficult to address these sex differences in epilepsy and in other neurological disease states. 9 ACCEPTED MANUSCRIPT Acknowledgements PT This work was supported by NIH grants NS052158 and NS051398] (to DSR). Dr. Reddy’s research work was supported by the CounterACT Program, National Institutes of Health, Office of the Director and the National Institute of Neurologic Disorders and Stroke [Grant U01 NS083460]. The author thanks Victoria Golub for reading the manuscript. RI References SC Amado, D., Cavalheiro, E. A., 1998. Hormonal and gestational parameters in female rats submitted to the pilocarpine model of epilepsy. Epilepsy Res. 32, 266-274. NU Atkinson, H.C., Leggett, J.D., Wood, S.A., Castrique, E.S., Kershaw, Y.M., Lightman, S.L., 2010. Regulation of the hypothalamic-pituitary-adrenal axis circadian rhythm by endocannabinoids is sexually diergic. Endocinol. 151, 3720-3727. MA Bäckström T., 1976. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurol. Scand. 54, 321–347. D Bäckström, T., Zetterlund, B., Blom, S., Romano, M., 1984. Effect of intravenous progesterone infusions on the epileptic discharge frequency in women with partial epilepsy. Acta Neurol. Scand. 69, 240–248. TE Bazan, A.C., Montenegro, M.A., Cendes, F., Min, L.L., Guerreiro, C.A., 2005. Menstrual cycle worsening of epileptic seizures in women with symptomatic focal epilepsy. Arg Neuro-Psiquiatria. 63, 751-756. AC CE P Belelli, D., Casula, A., Ling, A., Lambert, J.J., 2002. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacol. 43, 651-661. Benamer, H.T., Grosset, D.G., 2009. A systematic review of the epidemiology of epilepsy in Arab countries. Epilepsia 50, 2301-2304. Biagini, G., Baldelli, E., Longo, D., Pradelli, L., Zini, I., Rogawski, M.A., Avoli, M., 2006. Endogenous neurosteroids modulate epileptogenesis in a model of temporal lobe epilepsy. Exp. Neurol. 201, 519-524. Bonuccelli, U., Melis, G.B., Paoletti, A.M., Fioretti, P., Murri, L. Muratorio, A., 1989. Unbalanced progesterone and estradiol secretion in catamenial epilepsy. Epilepsy Res. 3, 100–106. Buckmaster, P.S. Haney, M.M., 2012. Factors affecting outcomes of pilocarpine treatment in a mouse model of temporal lobe epilepsy. Epilepsy Res. 102,153-159. Bujas, M., Pericic, D., Jazvinscak, M., 1997. Influence of gender and gonadectomy on bicuculline-induced convulsions on GABA-A receptors. Brain Res. Bull. 43, 411 – 416. Camfield, C.S., Camfield, P.R., 2009. Juvenile myoclonic epilepsy 25 years after seizure onset: a population-based study. Neurology 73, 1041–1045. Carver, C.M., Reddy, D.S., 2013. Neurosteroid interactions with synaptic and extrasynaptic GABA-A receptors: Regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology 230, 151-188. Carver, C.M., Reddy, D.S. 2016. Neurosteroid structure-activity relationships for functional activation of extrasynaptic δGABA-A receptors. J Pharmacol Exp Ther. 357,188-204 10 ACCEPTED MANUSCRIPT Carver, C.M., Wu, X., Gangisetty, O., Reddy, D.S. 2014. Perimenstrual-like hormonal regulation of extrasynaptic δ-containing GABAA receptors mediating tonic inhibition and neurosteroid sensitivity. J Neurosci. 34, 14181-14197. PT Chisari, M., Eisenman, L.N., Covey, D.F., Mennerick, S., Zorumski, C.F., 2010. The sticky issue of neurosteroids and GABA-A receptors. Trends Neurosci 33, 299–306. Christensen, J., Kjeldsen, M.J., Anderson, H., Friis, M.L., Sidenius, P., 2005. Gender differences in epilepsy 46, 956-960. RI Clayton, J.A., Collins, F.S., 2014. Policy: NIH to balance sex in cell and animal studies. Nature 509, 282-3. SC Cooke, B.M., Tabibnia, G., Breedlove, S.M., 1999. A brain sexual dimorphism controlled by adult circulating androgens. Proc. Natl. Acad. Sci. USA. 96, 7538-7540. NU Craig, C.R., 1966. Anticonvulsant activity of steroids: separability of anticonvulsant from hormonal effects. J Pharmacol. Exp. Ther. 153, 337–343. MA Dai, Y.J., Xu, Z.H., Feng, B., Xu, C.L., Zhao, H.W., Wu, D.C., Hu, W.W., Chen, Z., 2014. Gender difference in acquired seizure susceptibility in adult rats after early complex febrile seizures. Neurosci. Bull. 30, 913-922. Edwards, H. E., Burnham, W. M., Ng, M. M., Asa, S., MacLusky N.J., 1999. Limbic seizures alter reproductive function in the female rat. Epilepsia 40, 1370-1377. TE D El-Khayat, H.A., Soliman, N.A., Tomoum, H.Y., Omran, M.A., El-Wakad, A.S., Shatla. R., 2008. Reproductive hormonal changes and catamenial pattern in adolescent females with epilepsy. Epilepsia 49, 1619-1626. AC CE P Finn, D.A., Gee, K.W., 1994. The estrus cycle, sensitivity to convulsants and the anticonvulsant effect of a neuroactive steroid. J. Pharm. Exp. Ther. 271, 164–170. Frye, C.A., Reed, T.A., 1998. Androgenic neurosteroids: anti-seizure effects in an animal model of epilepsy. Psychoneuroendocrinology 23, 385–399. Gangisetty, O., Reddy, D.S., 2010. Neurosteroid withdrawal regulates GABA-A receptor α4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neurosci. 170, 865-880. Genazzani, A.R., Petraglia, F., Bernardi, F., Casarosa, E., Salvestroni, C., Tonetti, A., Nappi, R.E., Luisi, S., Palumbo, M., Purdy, R.H., Luisi, M., 1988. Circulating levels of allopregnanolone in humans: gender, age and endocrine influences. J. Clin. Endocrinol. Metab. 83, 2099– 2103. Gilad, R., Sadeh, M., Rapoport, A., Dabby, R., Lampl, Y., 2008. Lamotrigine and catamenial epilepsy. Seizure 17, 531-534. Gomes, P.X., de Oliveira, G.V., de Araújo, F.Y., de Barros Viana, G.S., de Sousa, F.C., Hyphantis, T.N., Grunberg, N.E., Carvalho, A.F., Macêdo, D.S., 2013. Differences in vulnerability to nicotine-induced kindling between female and male periadolescent rats. Psychopharmacol. 225, 115-126. Gulinello, M., Smith, S.S., 2003. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. J. Pharmacol. Exp. Ther. 305(2), 541-548. Harrison, N.L., Majewska, M.D., Harrington, J.W., Barker, J.L., 1987. Structure-activity relationships for steroid interactions with the -aminobutyric acidA receptor complex. J. Pharmacol. Exp. Ther. 241, 346-353. Hauser, W.A., 1997. Incidence and prevalence. In: Engel J, Jr., Pedley TA (Eds.), Epilepsy: A Comprehensive Textbook. Lippincott-Raven Publishers, Philadelphia, pp.47-57. 11 ACCEPTED MANUSCRIPT Herzog, A.G., 1991. Reproductive endocrine considerations and hormonal therapy for men with epilepsy. Epilepsia 32 (Suppl 6), S34-S37. Herzog, A.G., 2009. Hormonal therapies: progesterone. Neurotherap. 6, 383-391. Herzog, A.G., Klein, P. Ransil, B.J., 1997. Three patterns of catamenial epilepsy. Epilepsia 38, 1082–1088. PT Hesdorffer, D.C., Begley, C.E., 2013. Surveillance of epilepsy and prevention of epilepsy and its sequelae: lessons from the Institute of Medicine report. Curr Opin Neurol 26, 168-173. RI Hesdorffer, D.C., Logroscino, G., Benn, E.K., Katri, N., Cascino, G., Hauser, W.A., 2011. Estimating risk for developing epilepsy: A population-based study in Rochester, Minnesota. Neurol. 76, 23-27. SC Hosie, A.D., Wilkins, M.E., da Silva, H.M.A., Smart, T.G., 2006. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature 444, 486-489. NU Hosie, A.M., Clarke, L., da Silva, H., Smart, T.G., 2009. Conserved site for neurosteroid modulation of GABA-A receptors. Neuropharmacol. 56,149-154. MA Hosie, A.M., Wilkins, M.E., Smart, T.G., 2007. Neurosteroid binding sites on GABA-A receptors. Pharmacol. Ther. 116, 7-19. D Ikeda, H., Imai, K., Ikeda, H., Shigematsu, H., Takahashi, Y., Inoue, Y., Higurashi, N., Hirose, S., 2016. Characteristic phasic evolution of convulsive seizure in PCDH19-related epilepsy. Epileptic Disord. 18(1), 2633. TE Jacono, J.J., Robinson, J., 1987. The effects of estrogen, progesterone, and ionized calcium on seizures during the menstrual cycle in epileptic women. Epilepsia 28, 571–577. AC CE P Janszky, J., Schulz, R., Janszky, I., Ebner, A., 2004. Medial temporal lobe epilepsy: Gender differences. J. Neurol. Neurosurg. Psychi. 75, 773-775. Janz, D., 1998. Juvenile myoclonic epilepsy. Epilepsy: a comprehensive textbook. Lippincott Williams & Wilkins, Philadelphia. Kim, D.W., Lee, S.Y., Chung, S.E., Cheong, H.K., Jung, K.Y., Korean Epilepsy, S., 2014. Clinical characteristics of patients with treated epilepsy in Korea: A nationwide epidemiologic study. Epilepsia 55, 6775. Koppel, B.S., Harden, C.L., 2014. Gender issues in the neurobiology of epilepsy: a clinical perspective. Neurobiol Dis 72, 193-197. Landgren, S., Bäckström, T., Kalistratov, G., 1978. The effect of progesterone on the spontaneous interictal spike evoked by the application of penicillin to the cat's cerebral cortex. J. Neurol. Sci. 36, 119-133. Lawrence, C., Martin, B.S., Sun, C., Williamson, J., Kapur, J., 2010. Endogenous Neurosteroid Synthesis Modulates Seizure Frequency. Ann Neurol 67, 689–693. Li, H., Huguenard, J.R., Fisher, R.S., 2007. Gender and age differences in expression of GABA-A receptor subunits in rat somatosensory thalamus and cortex in an absence epilepsy model. Neurobiol. Dis. 25, 623-630. Logothetis, J., Harner, R., Morrel, F., 1959. The role of estrogens in catamenial exacerbation of epilepsy. Neurol. 9, 352-360. Lonsdale, D., 1982. Effect of thiamine tetrahydrofurfuryl disulfide on audiogenic seizures in DBA/2j mice. Dev. Pharmacol. Ther. 4, 28-36. 12 ACCEPTED MANUSCRIPT Lotte, J., Bast, T., Borusiak, P., Coppola, A., Cross, J.H., Dimova, P., 2016. Effectiveness of antiepileptic therapy in patients with PCDH19 mutations. Seizure. 35, 106-110. McHugh, J.C., Delanty, N., 2008. Epidemiology and classification of epilepsy: Gender comparisons. Int Rev Neurobiol 83, 11-26. PT Mejias-Aponte, C.A., Jimenez-Rivera, C.A., Segarra, A.C., 2002. Sex differences in models of temporal lobe epilepsy: role of testosterone. Brain Res. 944, 210–218. SC RI Mitev, Y.A., Darwish, M., Wolf, S.S., Holsboer, F., Almeida, O.F., Patchev, V.K., 2003. Gender differences in the regulation of 3 alpha-hydroxysteroid dehydrogenase in rat brain and sensitivity to neurosteroid-mediated stress protection. Neuroscience 120, 541-519. Nickel, J., Jokeit, H., Wunderlich, G., Ebner, A., Witte, O. W., Seitz, R. J., 2003. Gender-specific differences of hypometabolism in mtle: Implication for cognitive impairments. Epilepsia 44, 1551-1561. NU Ortiz-Gonzalez, X.R., Poduri, A., Roberts, C.M., Sullivan, J.E., Marsh, E.D., Porter, B.E., 2013. Focal cortical dysplasia is more common in boys than in girls. Epilepsy Behav. 27, 121-123. MA Panayiotopoulos, C.P., 2007. Epileptic syndromes and their treatment. Springer. Pericic, D., Bujas, M., 1997. Sex differences in bicuculline-induced convulsions: interaction with stress and ligands of benzodiazepine binding sites. Brain Res. 752, 279–284. D Pericic, D., Manev, H., Bujas, M., 1996. Gonadal hormones and picrotoxin-induced convulsions in male and female rats. Brain Res. 736, 174– 179. TE Persad, V., Cortez, M.A., Snead, O.C., 3rd 2002. A chronic model of atypical absence seizures: Studies of developmental and gender sensitivity. Epilepsy Res. 48, 111-119. AC CE P Persinger, M.A., Makarec, K., Bradley, J.C., 1988. Characteristics of limbic seizures evoked by peripheral injections of lithium and pilocarpine. Physiol. Behav. 44, 27-37. Perucca, P., Camfield, P., Camfield, C., 2014. Does gender influence susceptibility and consequences of acquired epilepsies? Neurobiol. Dis. 72, 125–130. Pesce, M.E., Acevedo, X., Bustamante, D., Miranda, H.E., Pinardi, G., 2000. Progesterone and testosterone modulate the convulsant actions of pentylenetetrazol and strychnine in mice. Pharmacol. Toxicol. 87, 116– 119. Quigg, M., Smithson, S.D., Fowler, K.M., Sursal, T., Herzog, A.G., Progesterone Trial Study Group., 2009. Laterality and location influence catamenial seizure expression in women with partial epilepsy. Neurol. 73, 223-227. Ravizza, T., Friedman, L.K., Moshe, S.L., Veliskova, J., 2003. Sex differences in GABAergic system in rat substantia nigra pars reticulate. In. J. Dev. Neurosci. 21, 245-254. Reddy, D.S., 2003. Is there a physiological role for the neurosteroid THDOC in stress-sensitive conditions? Trends Pharmacol. Sci. 24, 103-106. Reddy, D.S., 2004a. Anticonvulsant activity of the testosterone-derived neurosteroid 3-androstanediol. Neuroreport. 15, 515-518. Reddy, D.S., 2004b. Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3androstanediol and 17-estradiol. Neurosci. 129, 195-207. 13 ACCEPTED MANUSCRIPT Reddy, D.S., 2008. Mass spectrometric assay and physiological-pharmacological activity of androgenic neurosteroids. Neurochem Int. 52(4-5), 541-553. Reddy, D.S., 2009a. The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res. 85, 1-30. PT Reddy, D.S., 2009b. Steroid hormones and sex differences in seizure susceptibility. In: Encyclopedia of Basic Epilepsy Research (Editor, Philip Schwartzkroin), Vol 1. Oxford, Academic Press, 526-533. RI Reddy, D.S., 2010. Neurosteroids: Endogenous role in the human brain and therapeutic potentials. Prog. Brain Res. 186, 113-137. SC Reddy, D.S., 2011. Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy. Frontiers Endocrinol. 2, 1-11. NU Reddy, D.S., 2013a. Neuroendocrine aspects of catamenial epilepsy. Hormon. Behav. 63, 254–266. Reddy, D.S., 2013b. Role of hormones and neurosteroids in epileptogenesis. Front. Cell. Neurosci. 7(115), 120. MA Reddy, D.S., 2014. Neurosteroids and their role in sex-specific epilepsies. Neurobiol Dis. 72(Pt B), 198-209. Reddy, D.S., 2016a. Catamenial Epilepsy: Discovery of an Extrasynaptic Molecular Mechanism for Targeted Therapy. Front Cell Neurosci. 10 (101), 1-15. TE D Reddy, D.S., 2016b. Neurosteroids for the protection of humans against organophosphate toxicity. Annals of New York Academy of Sciences (in press). AC CE P Reddy, D.S., Estes, W.A. 2016. Clinical potential of neurosteroids for CNS disorders. Trends in Pharmacological Sciences 37(7), 543-561. Reddy, D.S., Clossen, B., Medi, H. 2016. Cellular and Molecular Mechanisms of Neuroinflammation in Epilepsy. Int J Pharm Sci Nanotech 9(in press). Reddy, D.S., Jian, K., 2010. The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors. J. Pharmacol. Exp. Ther. 334, 1031-1041. Reddy, D.S., Kim, H.Y., Rogawski, M.A., 2001. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia 42, 328-336. Reddy, D.S., Kuruba, R., 2013. Experimental models of status epilepticus and neuronal injury for evaluation of therapeutic interventions. Int. J. Mol. Sci. 14, 18284-18318. Reddy, D.S., Mohan, A., 2011. Development and persistence of limbic epileptogenesis are impaired in mice lacking progesterone receptors. J. Neurosci. 31, 650-658. Reddy, D.S., Ramanathan, G., 2012. Finasteride inhibits the disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Epilepsy Behav. 25, 92-97. Reddy, D.S., Rogawski, M.A., 2001. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia 42, 303-310. Reddy, D.S. Rogawski, M.A., 2009. Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics 6, 392-401. 14 ACCEPTED MANUSCRIPT Reddy, D.S., Zeng, Y.C., 2007. Effect of neurosteroid withdrawal on spontaneous recurrent seizures in a rat model of catamenial epilepsy. FASEB J. 21(6), A1179-A11179. Reddy, D.S., Castenada, D.A., O’Malley, B.W., Rogawski, M.A., 2004. Antiseizure activity of progesterone and neurosteroids in progesterone receptor knockout mice. J. Pharmacol. Exp. Ther. 310, 230-239. PT Reddy, D.S., Gangisetty, O., Briyal, S., 2010. Disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Neuropharmacol. 59, 573-581. RI Reddy, D.S., Rogawski, M.A., 2000a. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J. Pharmacol. Exp. Ther. 294, 909-915. SC Reddy, D.S., Rogawski, M.A., 2000b. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J. Pharmacol. Exp. Ther. 295, 1241-1248. NU Reddy, D.S., Rogawski, M.A., 2002. Stress-induced deoxycorticosterone-derived neuroactive steroids modulates GABAA receptor function and seizure susceptibility. J. Neurosci. 42, 3795-3805. MA Reddy, D.S., Rogawski, M.A., 2010. Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model. Epilepsy Res. 89, 254-260. Reddy, D.S., Woodward, R., 2004. Ganaxolone: a prospective overview. Drugs Future 29, 227-242. D Reich, C.G., Taylor, M.E., McCarthy, M.M., 2009. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav. Brain Res. 203, 264 –269. TE Rojas, A., Jiang, J., Ganesh, T., Yang, M.S., Lelutiu, N., Gueorguieva, P., Dingledine, R., 2014. Cyclooxygenase-2 in epilepsy. Epilepsia 55, 17–25. AC CE P Saalmann, Y.B., Kirkcaldie, M.T., Waldron, S., Calford, M.B., 2007. Cellular distribution of the GABA-A receptor-modulating 3α-hydroxy, 5α-reduced pregnane steroids in the adult rat brain. J. Neuroendocrinol. 19, 272–284. Santana, M.T., Jackowski, A.P., Britto Fdos, S., Sandim, G.B., Caboclo, L.O., Centeno, R.S., Carrete, H. Jr., Yacubian, E.M., 2014. Gender and hemispheric differences in temporal lobe epilepsy: A vbm study. Seizure 23, 274-279. Savic, I., Engel, J. Jr., 2014. Structural and functional correlates of epileptogenesis—does gender matter? Neurobiol. Dis. 70, 69 –73. Savic, I., Engel, J., Jr. 1998. Sex differences in patients with mesial temporal lobe epilepsy. J. Neurol. Neurosurg. Psychiatry. 65, 910-912. Scharfman, H.E., Goodman, J.H., Rigoulot, M.A., Berger, R.E., Walling, S.G., Mercurio, T.C., Stormes, K., Maclusky, N.J., 2005. Seizure susceptibility in intact and ovariectomized female rats treated with the convulsant pilocarpine. Exp. Neurol. 196, 73-86. Scharfman, H.E., Kim, M., Hintz, T.M., MacLusky, N.J., 2008. Seizures and reproductive function: insights from female rats with epilepsy. Ann. Neurol. 64(6), 687-697. Scharfman, H.E., Malthankar-Phatak, G.H., Friedman, D., Pearce, P., McCloskey, D.P., Harden, C.L., Maclusky, N.J., 2009. A rat model of epilepsy in women: A tool to study physiological interactions between endocrine systems and seizures. Endocrinol. 150, 4437-4442. 15 ACCEPTED MANUSCRIPT Scharfman, H.E., MacLusky, N.J., 2014. Sex differences in the neurobiology of epilepsy: a preclinical perspective. Neurobiol. Dis. 72(B), 180-192. Schwartz-Giblin, S., Korotzer, A., Pfaff, D.W., 1989. Steroid hormone effects on picrotoxin-induced seizures in female and male rats. Brain Res. 476Z, 240– 247. PT Sisodiya, S.M., Free, S.L., Thom, M., Everitt, A.E., Fish, D.R., Shorvon, S.D., 1999. Evidence for nodular epileptogenicity and gender differences in periventricular nodular heterotopia. Neurol. 52, 336-341. RI Smith, S.S., Shen, H., Gong, Q.H., Zhou, X., 2007. Neurosteroid regulation of GABA-A receptors: focus on the 4 and subunits. Pharmacol. Ther. 116, 58-76. SC Smith, C.D., Wright, L.K., Garcia, G.E., Lee, R.B., Lumley, L.A., 2015. Hormone-dependence of sarin lethality in rats: Sex differences and stage of the estrous cycle. Toxicol. Appl. Pharmacol. 287(3), 253-257. Sridharan, R., Murthy, B.N., 1999. Prevalence and pattern of epilepsy in India. Epilepsia 40, 631-636. NU Staley, K., 2015. Molecular mechanisms of epilepsy. Nature Neurosci. 18, 367-372. MA Tabatadze, N., Huang, G., May, R.M., Jain, A., Woolley, C.S., 2015. Sex differences in molecular signaling at inhibitory synapses in the hippocampus. J. Neurosci. 35(32), 11252–11265. D Tan, C., Shard, C., Ranieri, E., Hynes, K., Pham, D.H., Leach, D., Buchanan, G., 2015. Mutations of protocadherin 19 in female epilepsy (PCDH19-FE) lead to allopregnanolone deficiency. Hum. Mol. Genet. 24(18), 5250-5259. TE Tan, M., Tan, U., 2001. Sex difference in susceptibility to epileptic seizures in rats: importance of estrous cycle. Int. J. Neurosci. 108, 175– 191. AC CE P Taylor, I., Hodgson, B., Scheffer, I.E., Mulley, J., Berkovic, S.F., Dibbens, L., 2007. Is photosensitive epilepsy less common in males due to variation in X chromosome photopigment genes? Epilepsia 48, 1807-1809. Thomas, J., McLean, J.H., 1991. Castration alters susceptibility of male rats to specific seizures. Physiol. Behav. 49, 1177–1179. Tuveri, A., Paoletti, A.M., Orrù, M., Melis, G.B., Marotto, M.F., Zedda, P., Marrosu, F., Sogliano, C., Marra, C., Biggio, G., Concas, A., 2008. Reduced serum level of THDOC, an anticonvulsant steroid, in women with perimenstrual catamenial epilepsy. Epilepsia 49, 1221-1229. Twele, F., Tollner, K., Brandt, C., Loscher, W., 2016. Significant effects of sex, strain, and anesthesia in the intrahippocampal kainite mouse model of medial temporal lobe epilepsy. Epilepsy Behav. 55, 47-56. Valente, S.G., Naffah-Mazzacoratti, M.G., Pereira, M., Silva, I., Santos, N.F., Baracat, E.C., Cavalheiro, E.A., Amado, D., 2002. Castration in female rats modifies the development of the pilocarpine model of epilepsy. Epilepsy Res. 49, 181–188. van Luijtelaar, G., Onat, F.Y., Gallagher, M.J., 2014. Animal models of absence epilepsies: what do they model and do sex and sex hormones matter? Neurobiol. Dis. 72, 167–179. Veliskova, J., Moshe, S.L., 2001. Sexual dimorphism and developmental regulation of substantia nigra function. Ann. Neurol. 50, 596-601. Verrotti, A., Latini, G., Manco, R., De Simone, M., Chiarelli, F., 2007. Influence of sex hormones on brain excitability and epilepsy. J. Endocrinol. Invest. 30(9), 797-803. Vezzani, A., Aronica, E., Mazarati, A., Pittman, Q.J., 2013. Epilepsy and brain inflammation. Exp. Neurol. 244, 11–21. 16 ACCEPTED MANUSCRIPT VMDB Report. 2003. Search terms ‘‘epilepsy’’ in intact versus castrated dogs. The Veterinary Medical Database (www.vmdb.org). PT Werboff, L.H., Havlena, J., 1968. Audiogenic seizures in adult male rats treated with various hormones. Gen. Comp. Endocrinol. 3, 389–397. RI Wiley, J.L., Kendler, S.H., Burston, J.J., Howard, D.R., Selley, D.E., Sim-Selley, L.J., 2008. Antipsychoticinduced alterations in CB1 receptor-mediated G-protein signaling and in vivo pharmacology in rats. J. Neurosci. 55(7), 1183-1190. SC Wilson, M.A., Biscardi, R., 1997. Influence of gender and brain region on neurosteroid modulation of GABA responses in rats. Life Sci. 60(19), 1679-1691. NU Wohlfarth, K.M., Bianchi, M.T., Macdonald, R.L., 2002. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J. Neurosci. 22, 1541-1549. MA Wu, X., Gangisetty, O., Carver, C.M., Reddy, D.S., 2013. Estrous cycle regulation of extrasynaptic δcontaining GABA-A receptor-mediated tonic inhibition and limbic epileptogenesis. J. Pharmacol. Exp. Ther. 346, 146-160. Younus, I., Reddy, D.S. 2016. Seizure facilitating activity of the oral contraceptive ethinyl estradiol. Epilepsy Res. 121, 29-32. AC CE P TE D Zhang, X.M., Zhu, S.W., Duan, R.S., Mohammed, A.H., Winblad, B., Zhu, J., 2008 Gender differences in susceptibility to kainic acid-induced neurodegeneration in aged C57BL/6 mice. Neurotoxicol. 29(3), 406-412. 17 ACCEPTED MANUSCRIPT FIGURE LEGEND AC CE P TE D MA NU SC RI PT Figure 1. Schematic illustration of potential neuroendocrine basis of sex differences in neuronal excitability and seizure susceptibility. There are at least two distinct mechanisms by which steroid hormones, such as progesterone, deoxycorticosterone, testosterone and estrogens, affect neuronal excitability and seizure susceptibility: (i) binding to intracellular steroid receptors (SRs) (top panel) and (ii) metabolism to neuroactive steroids that can modulate ion channel receptors (bottom panel). Steroid hormones affect neural gene expression by classical genomic mechanisms via progesterone receptors (PRs), estrogen receptors (ERs), androgen receptors (ARs), glucocorticoid receptors (GCs) and mineralocorticoid receptors (MRs). Steroid hormones serve as precursors or intermediates for the biosynthesis of several neurosteroids via sequential enzymatic A-ring reductions in peripheral tissues and in the brain. Allopregnanolone and related neurosteroids bind and potentiate the GABA-A receptor function leading to enhanced inhibition in the brain. Some intermediate steroids can modulate both steroid receptors and ion channel receptors. Consequently, the neuroendocrine milieu provides many pathways for complex interaction between genomic and non-genomic actions of steroid hormones in the brain. 18 MA NU SC RI PT ACCEPTED MANUSCRIPT AC CE P TE D Figure 1 19 ACCEPTED MANUSCRIPT Revised Manuscript # PBB-D-16-00125 Invited Review Pharmacology, Biochemistry and Behavior (Special Issues: Behavioral Sex Differences; Editor: Dr. Alonso Fernández-Guasti) PT The Neuroendocrine Basis of Sex Differences in Epilepsy RI Doodipala Samba Reddy TE D MA NU Sex differences are apparent in epilepsy. The incidence of epilepsy is relatively higher in men than women. In women with epilepsy, seizures fluctuate according to menstrual cycle phases. The neuroendocrine basis of sex differences in epilepsy remains unclear. A mechanistic understanding is needed to optimize gender-specific therapies. AC CE P SC HIGHLIGHTS 20