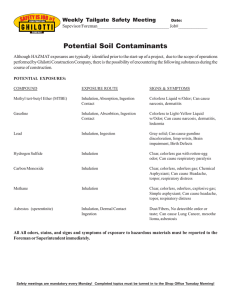
2 Review article Cyanide poisoning by fire smoke inhalation: a European expert consensus Kurt Anseeuwa,*, Nicolas Delvaub,*, Guillermo Burillo-Putzed, Fabio De Iacoe, Götz Geldnerf, Peter Holmströmg, Yves Lamberth and Marc Sabbec Downloaded from https://journals.lww.com/euro-emergencymed by BhDMf5ePHKav1zEoum1tQfN4a+kJLhEZgbsIHo4XMi0hCywCX1AWnYQp/IlQrHD33nx2+yvo/ecn2vosHJLy5x3rz4pdZ3J63oA+Z3xqIrQ= on 04/21/2019 Smoke inhalation is a common cause of cyanide poisoning during fires, resulting in injury and even death. In many cases of smoke inhalation, cyanide has increasingly been recognized as a significant toxicant. The diagnosis of cyanide poisoning remains very difficult, and failure to recognize it may result in inadequate or inappropriate treatment. Findings suggesting cyanide toxicity include the following: (a) a history of enclosed-space fire; (b) any alteration in the level of consciousness; (c) any cardiovascular changes (particularly inexplicable hypotension); and (d) elevated plasma lactate. The feasibility and safety of empiric treatment with hydroxocobalamin for fire smoke victims have been reported in the literature. On the basis of a literature review and a panel discussion, a group of European experts has proposed emergency management protocols for cyanide toxicity in fire smoke victims. European Journal of Emergency Medicine 20:2–9 c 2013 Wolters Kluwer Health | Lippincott Williams & Wilkins. European Journal of Emergency Medicine 2013, 20:2–9 Keywords: algorithm, cyanide inhalation, cyanide poisoning, guidelines, hydrogen cyanide, hydroxocobalamin, nitrites, smoke inhalation, smoke inhalation injury, thiosulphate a Department of Emergency Medicine, ZNA Stuivenberg, Antwerp, bDepartment of Emergency Medicine, Cliniques Universitaires Saint-Luc, Brussels, c Department of Emergency Medicine, University Hospital Gasthuisberg, Leuven, Belgium, dDepartment of Emergency Medicine, Hospital Universitario de Canarias, Tenerife, Spain, eDepartment of Emergency Medicine, A.S.L. 1 ‘Imperiese’, Imperia, Italy, fKlinikum Ludwigsburg, Ruprecht Karls University, Heidelberg, Germany, gHelsinki EMS, Helsinki University Hospital, Helsinki, Finland and hDepartment of Emergency Medicine, Versailles Hospital, Le Chesnay, France Correspondence to Kurt Anseeuw, MD, Department of Emergency Medicine, ZNA Stuivenberg, Lange Beeldekensstraat 267, B-2060 Antwerp, Belgium Tel: + 32 3 217 78 37; fax: + 32 3 217 75 37; e-mail: [email protected] *Kurt Anseeuw and Nicolas Delvau contributed equally to the writing of this article. Received 16 February 2012 Accepted 18 June 2012 Introduction Methodology During a fire, a complex toxic environment involving flames, heat, oxygen depletion, smoke and toxic gases is created. The inhalation of fire smoke, which contains a complex mixture of gases, seems to be the major cause of morbidity and mortality in fire victims. Although carboxyhaemoglobin levels have been shown to be an effective predictor of mortality, morbidity and duration of hospital stay in smoke inhalation, hydrogen cyanide (HCN) also seems to be a major source of concern. Cyanide also represents a major hazard to firefighters entering fires [1–5]. An international group of experts was independently formed to discuss the management of victims of cyanide inhalation by fire smoke in Europe and to define practical guidelines for prehospital and hospital care providers. The European Society for Emergency Medicine (EuSEM) endorsed this initiative and proposed several delegates knowledgeable on the subject (Council Meeting, March 2011). The aims were to describe and clarify diagnostic and therapeutic issues in the complex management of cyanide poisoning in smoke inhalation victims using a literature review following evidence-based techniques, and a two-round modified Delphi method to set up practical guidelines. Cyanide exists in several forms, including HCN gas, potassium and sodium cyanide salts, mercury, copper, gold and silver cyanide salts. HCN from fire smoke is probably the most common cause of acute cyanide poisoning in developed countries [1,6–8]. To our knowledge, only local and national guidelines on the management of cyanide poisoning by smoke inhalation exist [9]. Therefore, European experts met to formulate an algorithm to improve the recognition and management of cyanide poisoning and to enhance survival rates. Two algorithms were developed to improve the recognition and management of cyanide poisoning: a prehospital and a hospital-based algorithm. More information about the composition of the expert group and methodology can be found in the Supplemental digital content (see text, Supplemental digital content 1, http://links.lww.com/EJEM/A30, methodology; see text, Supplemental digital content 2, http://links.lww.com/EJEM/A31, bibliography). Literature Overview Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.euro-emergencymed.com). 0969-9546 c 2013 Wolters Kluwer Health | Lippincott Williams & Wilkins Is hydrogen cyanide formed during a fire? Several investigations have reported that carbon monoxide (CO) and HCN are major combustion products of DOI: 10.1097/MEJ.0b013e328357170b Cyanide inhalation Anseeuw et al. materials commonly found in domestic structures and high quantities of HCN are released, especially under pyrolyzing conditions at high temperatures and low oxygen content. In addition, the recycling of combustion products within a confined space increases the formation of HCN, and lower fire ventilation rates increase the formation of HCN by 6–10 times [10]. The presence and amount of specific smoke constituents vary within and between fires depending on the nature of the fire substrate, the rate of burning, the temperature of the fire and the ambient oxygen level [1,6]. CO is released in fires as a result of incomplete combustion of carbonaceous materials. During combustion of nitrogen-containing and carbon-containing substances, HCN may be generated by burning synthetics such as foam (polyurethane), acrylics, paints, nylon and plastics (polyacrylonitriles, polyacrylamides), as well as natural materials such as wool, silk, cotton, paper and wood. In general, the more nitrogen compounds, the greater the potential release of HCN during combustion. The increasingly widespread use of polymers makes it more likely that HCN will be encountered more frequently in smoke [1,6–8,10–12]. Epidemiology We must be aware that epidemiological data on cyanide levels are prone to biases. Because cyanide has a very short half-life and blood samples are rarely obtained within a short time, the cyanide concentrations measured are often erroneously low. The storage of sampled blood (storage temperature, time between sampling and assay) and the influence of various incident-specific and victimspecific factors (e.g. carboxyhaemoglobin saturation, methaemoglobin content) can also contribute towards the error [6,13]. Several case studies have reported different results. A documented hotel fire claimed 97 lives and injured more than 140 individuals. From fire dynamics modelling of this case, the principal cause of death was attributed to heat shock and oxygen depletion, explaining why burned victims had low and nonlethal carboxyhaemoglobin and blood cyanide levels. Interestingly, the burned victims had lower carboxyhaemoglobin values, indicating that smoke inhalation more likely contributed towards the deaths of nonburned victims [1,14]. 3 The Happy Land Social Club fire fatalities provided a unique opportunity to examine the effects on a homogeneous healthy population that was exposed to an identical smoke-filled environment. Of the decedents, 92% had carboxyhaemoglobin concentrations over 60% and 81% of the victims had toxic cyanide levels. However, no correlation was detected between carboxyhaemoglobin and blood cyanide levels [1,17]. In another study of 178 deaths because of burns, fire or smoke inhalation, positive results were found for carboxyhaemoglobin in 111 cases (72%) and cyanide in 88 cases (62%). Lethal levels (> 50%) were found for carboxyhaemoglobin in 44 cases (29%) and cyanide levels (> 1 mg/l) in 48 cases (35%). Significantly higher carboxyhaemoglobin and cyanide levels were observed in the intoxicated, very young, very old and death-at-thescene victims, and cyanide levels showed a significant correlation with carboxyhaemoglobin [18]. In contrast, in the New Jersey State database of fire deaths, only a weak correlation was observed between carboxyhaemoglobin and cyanide levels. However, elevated carboxyhaemoglobin levels were observed in 87% of patients and elevated cyanide levels in 75% of patients [2]. In an aircraft fire that killed 23 individuals out of 46, toxicological reports indicated carboxyhaemoglobin levels averaging 40% and cyanide levels averaging 2.33 mg/l in the 23 victims. In contrast, the average carboxyhaemoglobin level was 2.7% and the average cyanide level was 0.04 mg/l in the survivors [19]. In another aircraft fire, 54 individuals died of smoke inhalation and very low carboxyhaemoglobin levels were detected, but cyanide was detected in every victim, pointing out the primary role of HCN versus CO in these victims [1]. During an insurrection in a prison, 35 inmates died of smoke inhalation. Most (34%) showed cyanide levels of 2–4 mg/l and carboxyhaemoglobin levels of 5–10%, indicating that HCN (pyrolysis of polyurethane mattresses) was the main cause of death [20]. Low carboxyhaemoglobin levels were found in another hotel fire. Cyanide was also measured, but the results were too sketchy [1,15]. One-hundred and nine fire victims were studied by Baud and colleagues in the Paris area. The mean blood cyanide levels in the survivors (0.5 mg/l) and the deceased victims (3 mg/l) were significantly higher than those in the control group (0.1 mg/l). There was a marginal positive correlation between carboxyhaemoglobin and cyanide levels [1,13]. In a prospective study including 144 patients with either smoke inhalation or smoke exposure and 43 fire victims, elevated cyanide blood levels were found in both survivors (90%) and the deceased (100%). A statistically significant correlation was observed between cyanide and carboxyhaemoglobin levels [16]. In conclusion, CO and HCN play an important role in fire smoke toxicity, and HCN sometimes appears to be the primary cause of death. A limited correlation between CO and cyanide levels makes the prediction of cyanide concentration on the basis of the carboxyhaemoglobin level almost impossible. 4 European Journal of Emergency Medicine 2013, Vol 20 No 1 Pathophysiology Fire victims may be exposed to three potential injuries: burns, trauma and smoke inhalation. However, 60–80% of deaths at the fire scene are attributable to smoke inhalation. Smoke is a heterogeneous compound unique to each fire in both its chemical composition and its toxic features. The components of smoke that cause damage are: Smoke inhalation victims of a closed-space fire may therefore suffer from concurrent poisoning with HCN, CO and other asphyxiants. CO compromises arterial oxygen transport, whereas cyanide blocks oxygen utilization at the cellular level. The synergistic effect of combined CO and HCN poisoning has been reported [1,6,11]. (1) Heat, with a risk of glottis oedema. (2) Particulates, which are deposited in the airways according to their size. (3) Systemic toxins (up to 150 toxic molecules have been identified). (4) Respiratory irritants, causing intense and prolonged inflammatory reactions. Diagnosis In addition, smoke inhalation can also produce oxygen deprivation because of oxygen consumption by the fire and asphyxiant gases. Houeto and colleagues studied erythrocyte cholinesterase activity in 49 fire victims and found that it correlates with neither HCN nor CO. They believe that oxygen deprivation or as yet undetermined toxic gases may be responsible for this impairment [1,5,21,22]. HCN is a potent and rapidly acting poison. Signs and symptoms appear within seconds to minutes after exposure to moderate to high concentrations. The effect of a gas always depends on two parameters: the concentration and the duration of exposure. The cyanide ion, having a high affinity for metalloproteins, inhibits about 40 enzyme systems. It has an affinity in decreasing order for cobalt, ferric iron (Fe3+) in methaemoglobin, cytochrome oxidase and ferrous iron (Fe2+) in haemoglobin. It is thus principally fixed on the cytochrome aa3 oxidase, present in high concentrations in the mitochondria, inhibiting oxidative phosphorylation and resulting in a shift from aerobic to anaerobic metabolism. This anaerobic metabolism leads to cellular ATP depletion and lactic acidosis. Organs rich in cytochrome oxidase (brain, heart, liver) will be most affected. Our bodies can detoxify cyanide using several mechanisms including conversion into nontoxic thiocyanate by the enzyme rhodanese and conversion into cyanocobalamin by binding to hydroxocobalamin. These mechanisms are overwhelmed by exposure to all but very small concentrations of cyanide because of the depletion of sulphur donors [6–8,11,21,23–25]. The effects of CO are produced through different mechanisms: (1) Combining with haemoglobin to carboxyhaemoglobin and reducing the oxygen capacity; (2) Left shifting the oxygen–haemoglobin dissociation curve and thus impairing tissue oxygen availability; (3) Combining with myoglobin and thus impairing cardiac and skeletal oxygen availability; and (4) Inhibition of cytochrome enzymes [1,5,21]. Poisoning can be diagnosed on the basis of different criteria: clinical, biological or analytical. Early manifestations of cyanide toxicity reflect neurologic and respiratory stimulation, in an attempt to compensate for tissue hypoxia, and include giddiness, confusion, headache, vertigo and dizziness; nausea and vomiting; palpitations; and hyperventilation or shortness of breath. Later symptoms of acute cyanide poisoning reflect neurological, respiratory and cardiovascular depression arising from the inability to compensate for tissue hypoxia. Eventually, seizures, bradycardia, hypotension, coma, respiratory and cardiac arrest will ensue. Cardiovascular effects have been found in a canine model with two distinct phases: the first phase includes features of a hyperdynamic state, whereas the second phase follows a pattern consistent with cardiovascular failure. The importance of bradycardiac heart failure, with preservation of contractility, is introduced in this study. Cyanide also causes permanent neurological disability, ranging from various extrapyramidal syndromes to postanoxic vegetative states [4,11,12,23–31]. Besides clinical signs and symptoms, biological or analytical criteria can be used to predict cyanide poisoning. Different studies have found a significant correlation between plasma lactate and blood cyanide levels, both in fire victims and in victims of pure cyanide poisoning. A plasma lactate concentration of at least 90 mg/ dl is a sensitive and specific indicator of cyanide poisoning. In general, high lactate values are strongly suggestive of cyanide poisoning following smoke inhalation and may thus be used as a severity marker of cyanide toxicity. Unfortunately, lactic acidosis is nonspecific and is also found in a huge number of critical illnesses, even in CO poisoning. Serial lactate measurements can be useful in assessing the severity of cyanide poisoning. Theoretically, venous blood arteriolization should be present, but this finding has been poorly documented [11,13,26,32–34]. In contrast to CO, there is no rapid detection method for HCN in blood and it takes time to obtain analytical confirmation. Lab analysis will confirm the diagnosis, but treatment should start without waiting for the lab results. Because of its short half-life, a blood sample should be taken as soon as possible, if possible at the scene of the fire. The relationships between cyanide concentrations and the severity of symptoms are described as follows: concentrations in the range of 0.5–1 mg/l are considered Cyanide inhalation Anseeuw et al. as mild, between 1 and 2 mg/l as moderate, between 2 and 3 mg/l as severe and greater than 3 mg/l as lethal. Studies were performed to evaluate the cyanide breath concentration as an indicator of systemic cyanide poisoning [8,12,27,35,36]. Clinical smoke inhalation severity scores (composed of history, clinical examination and vital signs) seem to be the single strongest predictor of CO and HCN poisoning [4,5,21,28,29,31]. The diagnosis of cyanide poisoning is challenging because of the lack of pathognomonic symptoms and signs. To start with, diagnosis should be based on an index of suspicion, altered mental state and lactic acidosis. The suspicion of cyanide poisoning is heightened by the findings of hypotension combined with bradycardia [1,6,8,12,21,37]. Treatment HCN is fast acting; thus, the speedy and empiric treatment of fire smoke victims is critical for achieving successful intervention. Management involves removing the victim from the source of the cyanide, basic life support and administration of high-flow 100% oxygen. Supplemental oxygen is a crucial part of supportive care in cyanide poisoning, as it treats CO poisoning and may reactivate cyanide-inhibited mitochondrial enzymes. The use of hyperbaric oxygen for cyanide toxicity remains controversial: some studies have reported beneficial effects, whereas others have failed to report any improved outcome [11,21,35]. If necessary, additional supportive measures (e.g. blood pressure support, treatment of seizures, etc.) have to be adopted. Some report advocate aggressive cardiovascular support to increase hepatic clearance of cyanide by rhodanese, which may be successful in treating cyanide poisoning. Most authors, however, advise the administration of an antidote when suspecting cyanide toxicity. Fire smoke victims presenting with normal vital signs, a normal clinical examination and no lactate increase can be safely discharged home [4–8,11,21,29,38]. Three large families of antidotes exist: methaemoglobinforming agents [nitrites, 4-dimethylaminophenol (4-DMAP)], sulphur donors (thiosulphate) and cobalt compounds (dicobalt edetate, hydroxocobalamin) [11,12,25]. Nitrites reduce blood cyanide by forming methaemoglobin, which chelates cyanide to form cyanomethaemoglobin. 4-DMAP also neutralizes cyanide by inducing methaemoglobin. As cyanomethaemoglobin dissociates, it allows free cyanide to be converted into thiocyanate by rhodanese. Significant side-effects such as vasodilatation, hypotension, nephrotoxicity and haemolysis have been observed during treatment. 4-DMAP can generate methaemoglobin concentrations above 30%, exceeding the threshold value above which cardiovascular collapse and death can occur [6,12,25,37,39–43]. 5 Methaemoglobin generators have the potential to impair the oxygen-carrying capacity of haemoglobin by displacing oxygen from it. In smoke inhalation victims, with concomitant hypoxaemia of multiple causes and possible pulmonary injury, there is an obvious added risk associated with the formation of methaemoglobin. The additive oxygen-depriving effects of nitrites and CO can even be lethal, particularly in those with a pre-existing low cardiopulmonary reserve. This has been shown by the work of Moore and colleagues [7,21,37,39,42,43]. The enzyme rhodanese forms the less toxic thiocyanate, which is cleared renally by adding a sulphur atom to the cyanide. This reaction is accelerated more than three times by the administration of thiosulphate. Although the brain is highly susceptible to cyanide toxicity, thiosulphate does not readily penetrate cells and the mitochondria and its effectiveness is limited by its delayed onset of action, short half-life and small distribution volume. It is often used in conjunction with other rapid-acting agents rather than as a single antidote [11,25,39,42,44]. Cobalt compounds are rapid and powerful cyanide antidotes because of the strong affinity of cyanide to cobalt. Dicobalt edetate works by chelation of cyanide to form cobalticyanide. Adverse effects of dicobalt edetate include deleterious cardiovascular effects, urticaria, seizures and anaphylactic shock. The solution contains free cobalt ions, resulting in severe cobalt toxicity in the absence of cyanide. For those reasons, it is now recommended as a second-line antidote [25,42,44]. Hydroxocobalamin detoxifies by chelation of cyanide to form cyanocobalamin, or vitamin B12, which is excreted renally. Hydroxocobalamin is well tolerated, with only minor adverse effects (skin discoloration, chromaturia, urticaria, allergy) and it does not reduce oxygen-carrying capacity. Data from both human and animal studies shows that administration of hydroxocobalamin increases blood pressure, because of its nitric oxide-scavenging effects, and may be beneficial in counteracting hypotension in cyanide-poisoned patients. Pharmacokinetic and pharmacodynamic data suggest a predominantly extracellular partitioning of the antidote, but also intracellular and cerebrospinal fluid penetration. Because of its half-life, single-dose therapy, in sufficient quantity to bind all cyanide present, is likely to be adequate. When administered by an infusion, it dissolves almost immediately into the different tissue compartments, resulting in a rapid onset of action [12,25,40,42,44–52]. Prospective and retrospective human and animal data have described antidotal properties, with reported survival rates ranging from 42 to 72% for prehospital administration of hydroxocobalamin for suspected cyanide poisoning by fire smoke inhalation [25,37,40,48] Hydroxocobalamin can be used successfully in patients with cardiorespiratory instability, and also remains useful 6 European Journal of Emergency Medicine 2013, Vol 20 No 1 in patients with a cardiorespiratory arrest because of cyanide poisoning. Bebarta and colleagues compared hydroxocobalamin with thiosulphate and sodium nitrite with thiosulphate for cyanide poisoning treatment. Showing no difference in mortality, serum acidosis or serum lactate, the hydroxocobalamin group showed a faster return to the baseline mean arterial pressure than the thiosulphate group. This may have important clinical consequences. The recommended dose of hydroxocobalamin is 70 mg/kg (5 g). A second dose of 5 g should be given in case of cardiac arrest or persistent cardiovascular instability. Doses of 2.5 g have been shown to be insufficient to avoid neurological sequelae. The use of hydroxocobalamin can be combined with the administration of thiosulphate, but they should never be mixed in the same vial because of the formation of inefficient thiosulphatocobalamin [5,11,25,37,40,46–49,52–55]. The safety profile and rapid onset of action of hydroxocobalamin render prehospital or hospital empiric treatment of smoke inhalation-associated cyanide poisoning a reality [5,6,21,25,37,44]. Many agents have been explored as prophylactic cyanide antagonists. Cobinamide and sulfanegen seem to be Fig. 1 Cyanide poisoning by fire smoke inhalation Prehospital algorithm Smoke inhalation incident Evaluate: Fire in an enclosed space Duration of exposure Number of victims Administer O2 100% Severe poisoning GCS≤9 Intermediate poisoning GCS 10−13 and/or abnormal ABC No neurological and/or haemodynamic symptoms GCS≥14 Collect blood samples, if possible Hydroxocobalamin 5 g (70 mg/kg) i.v.a Hydroxocobalamin 5 g (70 mg/kg) i.v.b Monitoring Transfer to hospital Prehospital algorithm. GCS, Glasgow Coma Scale. a If cardiac arrest, administer 10 g of hydroxocobalamin. bIf several victims, begin with 2.5 g and top up to 5 g. Cyanide inhalation Anseeuw et al. Algorithms effective as intramuscular cyanide antidotes in mouse models, but further human testing is necessary [11,56]. On the basis of the above review, two algorithms for the treatment of smoke inhalation victims were developed. Although the use of any specific cyanide antidote in cyanide poisoning is unquestionable, the overall evidence for any specific antidote remains weak. With respect to practicality and safety advantages, the use of hydroxocobalamin seems most appropriate, but a large multicentre, randomized study is necessary to confirm this approach. Prehospital algorithm Because of the rapid onset of cyanide toxicity, we advocate the administration of an antidote as soon as possible, even prehospital. Although Europe has a culture of prehospital medical care, the first actors may differ from system to system. The prehospital algorithm was Fig. 2 Cyanide poisoning by fire smoke inhalation Hospital algorithm Smoke inhalation incident Other injuries (burns, trauma, etc.) Toxic effects of smoke Refer to other protocols Administer O2 100% History (type of fire, duration of exposure) Clinical examination Technical examination – imaging Lactates HbCO Arterial and venous blood gas Laboratory as needed CO poisoning 5. ECG 6. Thorax radiographs (only if necessary) 7. CN analysis (not required for acute treatment) Treat if clinical signs or HbCO>10% Follow local guidelines Other poisoning (acrolyne, phosgene, benzine, etc.) HOCo, 5 g High (70 mg/kg) i.v. Lactatea after 2 h Prehospital antidote Lactatea Normal Need for end-organ monitoring 1. 2. 3. 4. Normal (1) (2) (3) High Lactatea High HOCo, 5 g (70 mg/kg) i.v. High Normal High Normal If needed, consider transfer to reference centre Hospital algorithm. CO, carbon monoxide; CN, cyanide; HOCo, hydroxocobalamin. a Consider other causes (e.g. hypovolaemia). Lactatea after 2 h Cyanide poisoning Lactatea after 2 h Consider sodium thiosulphate No antidote 7 Normal Consider discharge 8 European Journal of Emergency Medicine 2013, Vol 20 No 1 designed in such a way that it could be used by paramedics, nurses and emergency physicians (Fig. 1). We propose dividing patients into three groups, depending on the degree of cyanide poisoning. We grade the degree of poisoning using the Glasgow Coma Scale (GCS) and vital signs. Severe poisoning is characterized by a GCS lower than 9 and/or severe haemodynamic instability (defined as heart rate below 40 bpm and/or systolic tension below 90 mmHg). Intermediate poisoning is described as a GCS from 10 to 13 with or without abnormal vital signs, and the absence of significant cyanide toxicity is characterized by a GCS at least 14. Using the GCS as the major determinant may result in overtreatment, but for us, this is the only reliable clinical parameter prehospitally to characterize cyanide poisoning. We advocate the administration of high-flow 100% oxygen to all smoke inhalation victims and transportation to an emergency department. Blood sampling for analytical analysis is proposed, as long as it does not delay patient treatment. Early administration of 70 mg/kg (5 g) hydroxocobalamin is advocated for all intermediate or severely poisoned patients. If a smoke inhalation victim presents with cardiorespiratory arrest or haemodynamical instability, 10 g hydroxocobalamin must be administered immediately, even during cardiopulmonary resuscitation. Giving the utmost importance of rapid treatment, we propose administering 2.5 g if multiple intermediate smoke inhalation victims are present at the scene, knowing that this is an insufficient dose, provided that the antidote is immediately topped up to 5 g at the hospital. All patients who received hydroxocobalamin or presented symptoms of cyanide poisoning should be admitted for end-organ monitoring. Asymptomatic patients, without antidotal therapy and negative lactates, can be safely discharged home after a short observation period. Conclusion There is growing evidence of cyanide toxicity in fire smoke victims presenting to the emergency department. Reducing mortality and morbidity attributed to cyanide inhalation primarily depends on early recognition and the timely administration of an antidote. Several specific cyanide antidotes are available, but given the practical and safety advantages, hydroxocobalamin is the preferred antidote. We developed two algorithms to improve the recognition and management of cyanide poisoning. Evaluation of these guidelines and the effect of implementation are planned. Acknowledgements Conflicts of interest Merck Serono Company supported the logistics of the Paris meeting. All experts received an honorarium from Merck Serono Company for taking part in the meeting. Professor Geldner received a honorarium from Merck Germany for giving lectures. He also participated at a National Advisory Board. For the remaining authors there are no conflicts of interest. References 1 2 3 4 Hospital algorithm Specific hospital management starts with an assessment of history and a clinical examination. A number of technical investigations have to be carried out, including lactate level, carboxyhaemoglobin level and arterial blood gas analysis. A blood sample for cyanide analytical determination also needs to be taken (Fig. 2). If not already done, the administration of high-flow 100% oxygen is mandatory. In a hospital setting, we propose using lactate levels as an indicator for cyanide toxicity. A lactate concentration at least 90 mg/dl must be considered as a positive indicator of cyanide poisoning. Victims of smoke inhalation presenting with elevated lactate levels should receive hydroxocobalamin 70 mg/kg (5 g), irrespective of whether or not they had already received a prehospital dose. If lactate levels remain elevated or if other signs of toxicity remain, further hydroxocobalamin should be administered, not exceeding the maximum dose of 10 g. When reaching the maximal dose of hydroxocobalamin, we propose the addition of sodium thiosulphate for further treatment. 5 6 7 8 9 10 11 12 13 14 15 Alarie Y. Toxicity of fire smoke. Crit Rev Toxicol 2002; 32:259–289. Barillo J, Goode R, Esch V. Cyanide poisoning in victims of fire: analysis of 364 cases and review of the literature. J Burn Care Rehabil 1994; 15:46–57. Jones J, McMullen MJ, Dougherty J. Toxic smoke inhalation: cyanide poisoning in fire victims. Am J Emerg Med 1987; 5:317–321. Mushtaq F, Graham CA. Discharge from the accident and emergency department after smoke inhalation: influence of clinical factors and emergency investigations. Eur J Emerg Med 2004; 11:141–144. Toon MH, Maybauer MO, Greenwood JE, Maybauer DM, Fraser JF. Management of acute smoke inhalation injury. Crit Care Resusc 2010; 12:53–61. Eckstein M, Maniscalco PM. Focus on smoke inhalation – the most common cause of acute cyanide poisoning. Prehosp Disaster Med 2006; 21:49–55. Guidotti T. Acute cyanide poisoning in prehospital care: new challenges, new tools for intervention. Prehosp Disaster Med 2005; 21:s40–s48. Kales SN, Christiani DC. Acute chemical emergencies. N Engl J Med 2004; 350:800–808. Duenas-Laita A, Burillo Putze G, Alonso JR, Bajo A, Climent B, Corral E, et al. Fundamentals in the clinical management of intoxication due to smoke inhalation. Emergencias 2010; 22:384–394. Tuovinen H, Blomqvist P. Modeling of hydrogen cyanide formation in room fires. Brandforsk project 321-011. SP Swedish National Testing and Research Institute, SP Report 2003, 10. Boras, Sweden. Gracia R, Shepherd G. Cyanide poisoning and its treatment. Pharmacotherapy 2004; 24:1358–1365. Lawson-Smith P, Jansen EC, Hyldegaard O. Cyanide intoxication as part of smoke inhalation – a review on diagnosis and treatment from the emergency perspective. Scand J Trauma Resusc Emerg Med 2011; 19:14. Baud FJ, Barriot P, Toffis V, Riou B, Vicaut E, Lecarpentier Y, et al. Elevated blood cyanide concentrations in victims of smoke inhalation. N Engl J Med 1991; 325:1761–1766. Levin BC, Rechani PR, Gurman JL, Landron F, Clark HM, Yoklavich MF, et al. Analysis of carboxyhemoglobin and cyanide in blood from victims of the Dupont Plaza hotel fire in Puerto Rico. J Forensic Sci 1990; 35:151–168. Birky MM, Malek DE, Paabo M. Study of biological samples obtained from victims of MGM Grand Hotel fire. J Anal Toxicol 1983; 7:265–271. Cyanide inhalation Anseeuw et al. 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 Silverman SH, Purdue GF, Hunt JL, Bost RO. Cyanide toxicity in burned patients. J Trauma 1988; 28:171–176. Gill JR, Goldfeder LB, Stajic M. The Happy Land homicides: 87 deaths due to smoke inhalation. J Forensic Sci 2003; 48:161–163. Yeoh MJ, Braitberg G. Carbon monoxide and cyanide poisoning in fire related deaths in Victoria, Australia. J Toxicol Clin Toxicol 2004; 42:855–863. Pane GA, Mohler SR, Hamilton GC. The Cincinnati DC-9 experience: lessons in aircraft and airport safety. Aviat Space Environ Med 1985; 56:457–461. Ferrari LA, Arado MG, Giannuzzi L, Mastrantonio G, Guatelli MA. Hydrogen cyanide and carbon monoxide in blood of convicted dead in a polyurethane combustion: a proposition for the data analysis. Forensic Sci Int 2001; 121:140–143. Rehberg S, Maybauer MO, Enkhbaatar P, Maybauer DM, Yamamoto Y, Traber DL. Pathophysiology management and treatment of smoke inhalation injury. Expert Rev Resp Med 2009; 3:283–297. Houeto P, Borron SW, Baud FJ, Muszynski J, Buisine A, Gourlain H. Assessment of erythrocyte cholinesterase activity in victims of smoke inhalation. J Toxicol Clin Toxicol 1999; 37:321–326. Pham JC, Huang DT, McGeorge FT, Rivers EP. Clarification of cyanide’s effect on oxygen transport characteristics in a canine model. Emerg Med J 2007; 23:152–156. Vogel SB, Sultan TR, Ten Eyck RP. Cyanide poisoning. Clin Toxicol 1981; 18:367–383. Megarbane B, Delahaye A, Goldgran-Tolédano D, Baud FJ. Antidotal treatment of cyanide poisoning. J Chin Med Assoc 2003; 66:193–203. Baud FJ, Borron SW, Megarbane B, Trout H, Lapostolle F, Vicaut E, et al. Value of lactic acidosis in the assessment of the severity of acute cyanide poisoning. Crit Care Med 2002; 30:2044–2050. Fortin JL, Desmettre T, Manzon C, Judic-Peureux V, Peugeot-Mortier C, Giocanti JP, et al. Cyanide poisoning and cardiac disorders: 161 cases. J Emerg Med 2010; 38:467–476. Shusterman D, Alexeeff G, Hargis C, Kaplan J, Sato R, Gelb A, et al. Predictors of carbon monoxide and hydrogen cyanide exposure in smoke inhalation patients. J Toxicol Clin Toxicol 1996; 34:61–71. Goh SH, Tiah L, Lim HC, Ng EK. Disaster preparedness: experience from a smoke inhalation mass casualty incident. Eur J Emerg Med 2006; 13:330–334. Rosenow F, Herholz K, Lanfermann H, Weuthen G, Ebner R, Kessler J, et al. Neurological sequelae of cyanide intoxication – the patterns of clinical, magnetic resonance imaging, and positron emission tomography findings. Ann Neurol 1995; 38:825–828. Woodson LC. Diagnosis and grading of inhalation injury. J Burn Care Res 2009; 30:143–145. Strickland A, Wang RY, Hoffman R, Goldfrank L. Blood cyanide concentrations after smoke inhalation. N Eng J Med 1992; 326:1362. Yeh MM, Becker CE, Arieff AI. Is measurement of venous oxygen saturation useful in the diagnosis of cyanide poisoning? Am J Med 1992; 93:582–583. Barillo DJ, Goode R, Rush BF, Lin RL, Freda A, Anderson EJ. Lack of correlation between carboxyhemoglobin and cyanide in smoke inhalation injury. Curr Surg 1986; 43:421–423. Lawson-Smith P, Jansen EC, Hilsted L, Hyldegaard O. Effect of hyperbaric oxygen therapy on whole blood cyanide concentrations in carbon monoxide intoxicated patients from fire accidents. Scand J Trauma Resusc Emerg Med 2010; 18:32. Stamyr K, Nord P, Johanson G. Washout kinetics of inhaled hydrogen cyanide in breath. Toxicol Lett 2008; 179:59–62. 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 9 Fortin JL, Giocanti JP, Ruttimann M, Kowalski JJ. Prehospital administration of hydroxocobalamin for smoke inhalation associated cyanide poisoning: 8 years of experience in the Paris Fire Brigade. Clin Toxicol 2006; 44:37–44. Houeto P, Borron SW, Sandouk P, Imbert M, Levillain P, Baud FJ. Pharmacokinetics of hydroxocobalamin in smoke inhalation victims. J Toxicol Clin Toxicol 1996; 34:397–404. Baskin SI, Horowitz AM, Neally EW. The antidotal action of sodium nitrite and sodium thiosulfate against cyanide poisoning. J Clin Pharmacol 1992; 32:368–375. Borron SW, Baud FJ, Barriot P, Imbert M, Bismuth C. Prospective study of hydroxocobalamin for acute cyanide poisoning in smoke inhalation. Ann Emerg Med 2007; 49:794–801. Hall AH, Kulig KW, Rumack BH. Suspected cyanide poisoning in smoke inhalation: complications of sodium nitrite therapy. J Toxicol Clin Exp 1989; 9:3–9. Hall AH, Saiers J, Baud F. Which cyanide antidote? Crit Rev Toxicol 2009; 39:541–552. Moore SJ, Norris JC, Walsh DA, Hume AS. Antidotal use of methemoglobin forming cyanide antagonists in concurrent carbon monoxide/cyanide intoxication. J Pharmacol Exp Ther 1987; 242:70–73. Hall AH, Dart R, Bogdan G. Sodium thiosulfate or hydroxocobalamin for the empiric treatment of cyanide poisoning? Ann Emerg Med 2007; 49:806–813. Astier A, Baud FJ. Complexation of intracellular cyanide by hydroxocobalamin using a human cellular model. Hum Exp Toxicol 1996; 15:19–25. Bebarta VS, Tanen DA, Lairet J, Dixon PS, Valtier PS, Bush A. Hydroxocobalamin and sodium thiosulfate versus sodium nitrite and sodium thiosulfate in the treatment of acute cyanide toxicity in a swine (Sus scrofa) model. Ann Emerg Med 2010; 55:345–351. Borron SW, Stonerook M, Reid F. Efficacy of hydroxocobalamin for the treatment of acute cyanide poisoning in adult beagle dogs. Clin Toxicol 2006; 44 (Suppl 1):5–15. Borron SW, Baud F, Megarbane B, Bismuth C. Hydroxocobalamin for severe acute cyanide poisoning by ingestion or inhalation. Am J Emerg Med 2007; 25:551–558. Forsyth JC, Mueller PD, Becker CE, Osterloh J, Benowitz NL, Rumack BH, et al. Hydroxocobalamin as a cyanide antidote: safety, efficacy, and pharmacokinetics in heavily smoking normal volunteers. J Toxicol Clin Toxicol 1993; 31:277–294. Gerth K, Ehring T, Braendle M, Schelling P. Nitric oxide scavenging by hydroxocobalamin may account for its hemodynamic profile. Clin Toxicol 2006; 44 (Suppl 1):29–36. Riou B, Gerard JL, La Rochelle CD, Bourdon R, Berdeaux A, Giudicelli JF. Hemodynamic effects of hydroxocobalamin in conscious dogs. Anesthesiology 1991; 74:552–558. Shepherd G, Velez LI. Role of hydroxocobalamin in acute cyanide poisoning. Ann Pharmacother 2008; 42:661–669. Uhl W, Nolting A, Golor G, Rost KL, Kovar A. Safety of hydroxocobalamin in healthy volunteers in a randomized, placebo-controlled study. Clin Toxicol 2006; 44:17–28. Hall AH, Rumack BH. Hydroxocobalamin/sodium thiosulfate as a cyanide antidote. J Emerg Med 1987; 5:115–121. Johnson JD, Meisenheimer TL, Isom GE. Cyanide-induced neurotoxicity: role of neuronal calcium. Toxicol Appl Pharmacol 1986; 84:464–469. Chan A, Crankshaw DL, Monteil A, Patterson SE, Nagasawa HT, Briggs JE, et al. The combination of cobinamide and sulfanegen is highly effective in mouse models of cyanide poisoning. Clin Toxicol (Phila) 2011; 49:366–373.