FORMULARIO Y CONSTANTES FÍSICAS
Anuncio
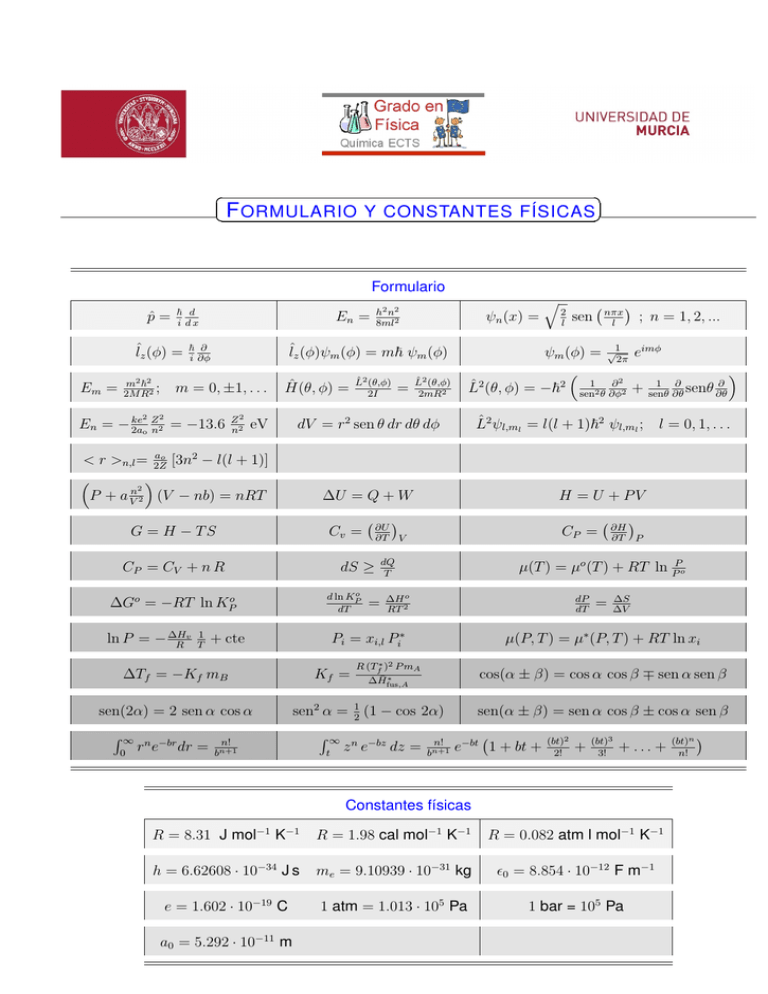
F ORMULARIO Y CONSTANTES FÍSICAS Formulario p̂ = ~ d i dx ˆlz (φ) = Em = m2 ~2 ; 2M R2 2 En = m = 0, ±1, . . . 2 ke Z En = − 2a 2 = −13.6 o n Z2 n2 Ĥ(θ, φ) = eV ; n = 1, 2, ... ψm (φ) = √12π eimφ 1 1 ∂ ∂ ∂2 2 2 L̂ (θ, φ) = −~ sen2 θ ∂φ2 + senθ ∂θ senθ ∂θ L̂2 (θ,φ) 2mR2 ∂U ∂T V dS ≥ o d ln KP dT ∆Go = −RT ln KPo = CP = dQ T Kf = ∆H o RT 2 dP dT n! R∞ R (Tf∗ )2 P mA t = ∆S ∆V cos(α ± β) = cos α cos β ∓ sen α sen β ∗ ∆H̄fus,A z n e−bz dz = µ(P, T ) = µ∗ (P, T ) + RT ln xi sen2 α = 12 (1 − cos 2α) bn+1 ∂H ∂T P µ(T ) = µo (T ) + RT ln PPo Pi = xi,l Pi∗ + cte ∆Tf = −Kf mB 0 H = U + PV CP = CV + n R rn e−br dr = nπx l sen ∆U = Q + W Cv = sen(2α) = 2 sen α cos α = 2 l L̂2 ψl,ml = l(l + 1)~2 ψl,ml ; l = 0, 1, . . . G = H − TS R∞ L̂2 (θ,φ) 2I q dV = r2 sen θ dr dθ dφ ao < r >n,l = 2Z [3n2 − l(l + 1)] 2 P + a Vn 2 (V − nb) = nRT 1 T ψn (x) = ˆlz (φ)ψm (φ) = m~ ψm (φ) ~ ∂ i ∂φ v ln P = − ∆H R h2 n2 8ml2 n! bn+1 sen(α ± β) = sen α cos β ± cos α sen β e−bt 1 + bt + (bt)2 2! + (bt)3 3! + ... + Constantes físicas R = 8.31 J mol−1 K−1 R = 1.98 cal mol−1 K−1 R = 0.082 atm l mol−1 K−1 h = 6.62608 · 10−34 J s me = 9.10939 · 10−31 kg 0 = 8.854 · 10−12 F m−1 e = 1.602 · 10−19 C 1 atm = 1.013 · 105 Pa 1 bar = 105 Pa a0 = 5.292 · 10−11 m (bt)n n! Factores radiales de los orbitales atómicos 3/2 e−Zr/ao R1s = 2 aZo 3/2 Zr R2s = √12 aZo 1 − 2a e−Zr/2ao o 5/2 1 √ re−Zr/2ao R2p = 2 6 aZo 3/2 2Z 2 r2 1 − 2Zr + e−Zr/3ao R3s = 3√2 3 aZo 3ao 27a2o 3/2 8√ Z Zr Z 2 r2 R3p = 27 6 ao − 6a2 e−Zr/3ao ao o Armónicos esféricos. Yl,m (θ, φ) = √12π Sl,m (θ)eimφ Rπ |Sl,m |2 sen θdθ = 1 0 √ l=0: S0,0 = 12 2 √ l=1: S1,0 = 12 6 cos θ √ S1,±1 = 12 3 sen θ √ l=2: S2,0 = 14 10(3 cos2 θ − 1) √ S2,±1 = 12 15 sen θ cos θ √ S2,±2 = 14 15 sen2 θ √ l=3: S3,0 = 34 14( 35 cos3 θ − cos θ) √ S3,±1 = 18 42 sen θ(5 cos2 θ − 1) √ S3,±2 = 14 105 sen2 θ cos θ √ S3,±3 = 18 70 sen3 θ
