Study of Mucociliary Transport and Nasal Ciliary Ultrastructure in
Anuncio

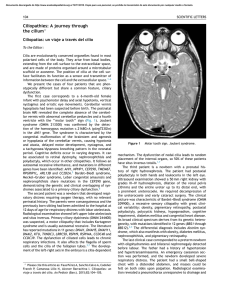
Documento descargado de http://www.archbronconeumol.org el 19/11/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato. ORIGINAL ARTICLES Study of Mucociliary Transport and Nasal Ciliary Ultrastructure in Patients With Kartagener’s Syndrome M. Armengot Carceller,a C. Carda Batalla,b A. Escribano,c and G.J. Samperd a Servicio de Otorrinolaringología, Hospital General Universitario, Facultad de Medicina, Valencia, Spain. Departamento de Patología, Facultad de Medicina, Valencia, Spain. c Servicio de Pediatría, Hospital Clínico Universitario, Facultad de Medicina, Valencia, Spain. d Servicio de Neumología, Hospital General Universitario, Facultad de Medicina, Valencia, Spain. b OBJECTIVE: Kartagener’s syndrome (KS) is a clinical variant of primary ciliary dyskinesia involving situs inversus associated with chronic airway infections. The ciliary defect associated with this syndrome is the absence of dynein arms. The aim of this study was to evaluate mucociliary transport and ciliary ultrastructure in 14 patients with KS. PATIENTS AND METHODS: We studied nasal mucociliary transport using a radioisotopic technique and ciliary ultrastructure in 14 patients with KS. RESULTS: Thirteen patients had mucociliary stasis and 1 had severely slowed transport (1.3 mm/min). Four patients (29%) had cilia with normal dynein arms, 2 patients (14%) had short inner dynein arms, and 8 patients (57.1%) had total absence of inner and outer dynein arms. CONCLUSIONS: We conclude that the typical clinical presentation, together with altered mucociliary transport as identified by an isotopic technique, is diagnostic of KS, even when the ciliary ultrastructure is normal. KS is clinically homogenous and morphologically heterogenous. Key words: Primary ciliary dyskinesia. Situs inversus. Mucociliary function. Estudio del transporte mucociliar y de la ultraestructura ciliar nasales en pacientes con síndrome de Kartagener OBJETIVO: El síndrome de Kartagener (SK) es una variante clínica de la discinesia ciliar primaria que asocia a las infecciones crónicas de las vías respiratorias un situs inversus. La ausencia de brazos de dineína ha sido el defecto ciliar asociado a este síndrome. El objeto de este trabajo es el estudio del transporte mucociliar y de la ultraestructura ciliar en 14 pacientes con SK. PACIENTES Y MÉTODOS: Hemos estudiado el transporte mucociliar nasal, mediante una técnica radioisotópica, y la ultraestructura ciliar en 14 pacientes con SK. RESULTADOS: En 13 pacientes había estasis mucociliar y en uno, un transporte muy enlentecido (1,3 mm/min). Mostraban cilios con brazos de dineína normales 4 pacientes (29%); brazos internos de dineína cortos, 2 pacientes (14%), y ausencia completa de brazos internos y externos de dineína, 8 casos (57,1%). CONCLUSIONES: Concluimos que la presentación clínica típica junto con un transporte mucociliar alterado, objetivado con una técnica isotópica, es diagnóstica del SK, aunque la ultraestructura ciliar sea normal. El SK es clínicamente homogéneo y morfológicamente heterogéneo. Palabras clave: Discinesia ciliar primaria. Situs inversus. Función mucociliar. Introduction The clinical triad of chronic sinusitis, bronchiectasis, and situs inversus is known as Kartagener’s syndrome (KS).1 First described at the beginning of the 20th century, KS is now recognized as a clinical variant of primary ciliary dyskinesia (PCD).2,3 PCD is an autosomal recessive disorder characterized by inefficient or absent mucociliary clearance.4 The coexistence of PCD and situs inversus is called KS and occurs in 50% of PCD Correspondence: Dr. M. Armengot Carceller. Mediterrani, 33. 46134 Foios. Valencia. España. E-mail: [email protected] Manuscript received January 23, 2004. Accepted for publication May 24, 2004. patients.5 Situs inversus can be defined as the random distribution of internal organs during embryogenesis, probably due to the absence of the ciliary activity that is responsible for normal organ distribution.5 Clinically, PCD is characterized by chronic infections of the upper and lower airways—including the middle ear—from birth. Bronchiectasis affects more than 80% of patients with PCD. However, since the frequency of bronchiectasis increases with age, very young patients are unaffected.6 Chronic sinusitis is present in all cases, as is hypoplasia of the paranasal sinuses—especially the frontal sinuses, caused by the eutrophic defect of a diseased mucosa.4 Infertility, caused by immotile spermatozoa, affects 80% of males with PCD: the axonemal ultrastructure of sperm flagella Arch Bronconeumol. 2005;41(1):11-5 11 Documento descargado de http://www.archbronconeumol.org el 19/11/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato. ARMENGOT CARCELLER M, ET AL. STUDY OF MUCOCILIARY TRANSPORT AND NASAL CILIARY ULTRASTRUCTURE IN PATIENTS WITH KARTAGENER’S SYNDROME Figure 1. Cross section of a ciliary axoneme in which the absence of dynein arms can be observed. The arrow indicates the empty space where the dynein arms should be. This is probably a case with completely immotile cilia. Corresponds to case 14 of the Table. is similar to that of respiratory cilia. However, because the polypeptide composition of cilia is different from that of flagella, not all males are infertile.7 Due to ciliary dysfunction in the fallopian tube, female fertility is also affected, although to a lesser degree.8,9 Ciliary dysfunction may also be the cause of some ectopic pregnancies in these patients.10,11 The absence of dynein arms was the first ciliary defect associated with KS.11 This deficiency can involve the inner or outer dynein arms—or both—and affects from 70% to 80% of patients.12 Other defects of the ciliary ultrastructure associated with KS include absence of radial spokes, ciliary disorientation, and ciliary transposition.6 This extensive morphological variety comes about because the ciliary axoneme is a biological structure consisting of at least 130 distinct polypeptides.5 Nevertheless, cases of patients with PCD and KS with normal ciliary ultrastructure have been reported.13-16 The aim of this paper was to present the results of a study of nasal mucociliary transport and ciliary ultrastructure in 14 patients with KS. Patients and Methods Fourteen patients with a mean age of 21 years (range, 1-49 years) diagnosed with KS were included in the study. Five of the patients were females. High-resolution computed tomography was used to diagnose disease in the maxillofacial area and the lungs. Male adults underwent sperm analysis. The study included 2 sisters (cases 2 and 3) who had no other siblings and 3 brothers (cases 11, 12, and 13) who had a healthy sister. Nasal mucociliary transport was assessed using a radioisotopic technique involving serum albumin labeled with metastable technetium 99 (Tc 99m) according to the following standard protocol: 0.01 mL (1 drop) of macroaggregated albumin was labeled with Tc 99m (with a radioactivity of 25 µCi at the surface of the drop) as a tracer.17 A micropipette was used to place the drop in the posterior 12 Arch Bronconeumol. 2005;41(1):11-5 area of the head of the inferior nasal concha. Eighty percent of the aggregates ranged in size from 60 µm to 80 µm, and none exceeded 100 µm. Less than 3.5 mg were deposited in total. The test lasted 15 minutes and was performed at room temperature (25°C) with a relative humidity of 64%. After completion of the procedure, the nasal cavity was rinsed with physiological saline solution. All tests were performed only after at least 5 weeks had passed with no signs of concurrent infection. The velocity of nasal mucociliary transport was calculated according to the following parameters: a) initial position of the tracer; b) final position of the tracer; c) time elapsed; and d) velocity (the distance traveled divided by the time elapsed). All nasal mucociliary transport velocities greater than 4 mm/min were considered normal.18 Biopsy samples were obtained from the middle nasal concha using endoscopically-guided curettage after applying topical lidocaine as an anesthesia. The tissue samples were fixed by immersion in 0.03 M phosphate-buffered 2.5% glutaraldehyde for 1 hour. They were then rinsed in the same buffer for 30 minutes and fixed in 1% osmium tetroxide for 1 hour. The samples were dehydrated in graded acetone solutions and embedded in Epon. Semi-thin sections from all the samples were stained with toluidine blue and the most representative areas were selected to make the ultra-thin sections. After staining with uranyl acetate and lead citrate, the ultra-thin sections were examined with an electron microscope. The following characteristics of the ciliary axonemes were evaluated: dynein arms (inner and outer), the central pair of microtubules (presence or absence and location), radial spokes (presence or absence), peripheral microtubules (position and number), compound cilia, ciliary orientation (relative to the orientation of the central pair), and other factors (ciliary membrane evaginations and incomplete axonemes). We studied at least 100 ciliary cross sections per patient. Dynein arms (inner, outer, or both) were considered absent when the mean number of dynein arms counted in all cross sections was less than 2 per cross section.19 Ciliary orientation was considered normal if deviation from the ciliary axis was less than 28°.20 Results Thirteen patients had the classic clinical triad of chronic sinusitis, chronic bronchitis with bronchiectasis, and situs inversus. Bronchiectasis was not present in case 14 given the patient’s young age (1 year). Sperm analysis revealed that all adult males (cases 5, 7, 10, and 11) had immotile sperm. Nine patients, 4 of whom did not have situs inversus, reported a family history of chronic respiratory disease The other 5 cases included 2 sisters (cases 2 and 3) without other siblings and 3 brothers (cases 11, 12, and 13) who had a healthy sister. Mucociliary transport was abnormal in all patients. Mucociliary stasis (absence of mucociliary transport) was present in 13 cases and 1 had severely slowed transport (1.3 mm/min) (Table). Eight patients (57.1%) had total absence of dynein arms (Figure 1). The dynein arms were normal in 4 patients (28%) (Figure 2), and in 2 cases (14%) the outer arms were normal while the inner arms were Documento descargado de http://www.archbronconeumol.org el 19/11/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato. ARMENGOT CARCELLER M, ET AL. STUDY OF MUCOCILIARY TRANSPORT AND NASAL CILIARY ULTRASTRUCTURE IN PATIENTS WITH KARTAGENER’S SYNDROME Figure 2. Cross section of ciliary axonemes with normal dynein arms. See arrows. Atypical case. Molecular or enzymatic alteration. Corresponds to case 1 of the Table. Figure 3. Cross section in which inner dynein arms are missing. See arrows. This is probably a case of inefficient ciliary transport (dyskinesia). Corresponds to case 9 of the Table. abnormally short (Figure 3). Although the actual incidence was quite low, nearly all patients had abnormal peripheral microtubule pairs (supernumerary microtubules) and compound cilia. Ciliary orientation was normal in all cases. A few patients had central pair abnormalities, ciliary membrane evaginations (Figure 4), or incomplete axonemes. The radial spokes were visible in all patients (Table). Discussion PCD should be considered in the differential diagnosis of patients who have had chronic infections of the upper and lower airways since birth.4 The likelihood of a suspected diagnosis of PCD is greater in cases with chronic infections and situs inversus. However, the diagnosis should first be confirmed so that early intervention can be initiated to avoid permanent sequelae, Figure 4. Cross section of ciliary axonemes with presence of dynein and ciliary membrane evaginations secondary to the chronic infection. Typical finding from secondary ciliary dyskinesia. See arrows. Corresponds to case 7 of the Table. TABLE Nasal Mucociliary Transport and Ciliary Ultrastructure in Patients With Kartagener Syndrome* Case Age/Sex Mucociliary Transport Dynein Arms Central Pair Radial Spokes Peripheral Microtubules Compound Cilia Ciliary Orientation Others Findings 1 2 3 4 5 6 7 8 9 10 11 12 13 14 49/F 16/F 18/F 13/M 41/M 17/F 38/M 13/M 13/F 38/M 32/M 12/M 15/M 1/M Stasis Stasis Stasis Stasis Stasis 1.3 mm/min Stasis Stasis Stasis Stasis Stasis Stasis Stasis Stasis N A A A A N N N Sh/I Sh/I A A A A N N N N E=20% N N N N N N N N E=1% N N N N N N N N N N N N N N S<2% S<1% S<1% S=8% S=20% S<1% S<1% No S<1% S=2% S<1% S<1% S<1% S=2% <1% No No <1% 4% <1% 10% No <1% <1% 10% 5% 5% 14% N N N N N N N N N N N N N N No No CE<1% No CE<1%; IA<1% No CE=2% CE<1% No No No No No No *F indicates female; M, male; N, normal; A, absent; Sh, short; I, inner dynein arms; S, supernumerary; E, eccentric; CE, ciliary membrane evaginations; IA, incomplete ciliary axoneme. Arch Bronconeumol. 2005;41(1):11-5 13 Documento descargado de http://www.archbronconeumol.org el 19/11/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato. ARMENGOT CARCELLER M, ET AL. STUDY OF MUCOCILIARY TRANSPORT AND NASAL CILIARY ULTRASTRUCTURE IN PATIENTS WITH KARTAGENER’S SYNDROME especially chronic sinusitis and bronchiectasias. Peripheral airway obstruction caused by the retained secretions leads to recurrent pneumonitis with associated microatelectasis, which will progress to bronchiectasis and, finally, to pulmonary fibrosis with its associated detrimental effects on lung and heart function.21 Appropriate early intervention may delay the emergence of bronchiectasis, which is not an intrinsic manifestation of the disease but rather the result of the chronic bronchial infection, as we observed in our patients.6 Likewise, the term chronic sinusitis has been replaced by chronic rhinosinusitis in accordance with recently agreed definitions.22 Therefore, the classic triad that defines KS should be modified to recognize that the combination of chronic upper and lower airway infections from birth with situs inversus has the same pathophysiological and clinical significance as the triad. The ultrastructural defects of the ciliary axoneme that we assessed in this study are the ones most often observed in PCD: absence of inner and/or outer dynein arms, radial spoke defects, ciliary transposition, and ciliary disorientation. In some atypical cases the ciliary ultrastructure is normal.6 The absence of central microtubules and missing nexin links have been described as possible congenital ciliary defects, although definitive confirmation of this classification is still needed.23,24 The complete absence of dynein arms is associated with immotile cilia. Other ciliary defects are associated with abnormal and inefficient ciliary beat patterns.25 Immotile cilia and cilia that have an inefficient beat produce the same result: stasis of respiratory secretions. Findings from our study indicate that ciliary ultrastructure is normal in approximately 30% of patients with KS. This important fact has previously been reported by other authors, although in smaller case series with a lower percentage of affected patients (approximately 3%).13-16,26,27 In cases in which the ciliary ultrastructure is normal, poor ciliary function is probably caused by a molecular or enzymatic deficiency.4 This is why some expert panels argue that abnormalities of the ciliary ultrastructure should be excluded from the definition of KS.27 The peripheral microtubule alterations that we found, as well as the presence of compound cilia and ciliary membrane evaginations, are characteristic of ciliary dyskinesia secondary to chronic infection of the epithelium. An eccentric central pair is the manifestation of a radial spoke deficiency and should probably be considered a primary (congenital) abnormality. In our study, 2 patients had central pair involvement, although the percentage of affected axonemes was insignificant. Given that this anomaly coincided with an absence of dynein arms, this finding is of doubtful pathological significance.28-30 Thus, defects in the ciliary ultrastructure, in the form of dynein deficiencies, should be considered diagnostic of KS in patients with chronic infections of the upper and lower airways and situs inversus. Nevertheless, a normal ultrastructure does not rule out KS. 14 Arch Bronconeumol. 2005;41(1):11-5 Curettage offers the ability to obtain a large sample of cells and allows us to select from the specific areas of the nasal mucosa with a high ciliary density (that is, the middle nasal concha). This is why many authors prefer this method.6 The radioisotopic technique using macroaggregated albumin, which remain on the surface of the mucus gel layer, is very useful for conventional as well as early diagnosis of PCD.31 It can even be performed on newborn babies because the radioactive dosage involves no risk to the organism.32 It is objective, precise, and reproducible.18 Although a negative result from this single test is not diagnostic of PCD, it is diagnostic when confirmed by the clinical presentation and ultrastructural study. A normal result rules out PCD.4 The more widely used saccharine test, despite its value, has serious disadvantages: it is subjective; it cannot be used with children; and the solubility of saccharine permits it to diffuse into the periciliary layer of the mucus, where transport is slow and irregular.18 In conclusion, the combination of chronic upper and lower airway infections from birth, together with situs inversus and nasal mucociliary stasis confirmed by an isotopic method, should be considered a definitive diagnosis for KS, even if the ciliary ultrastructure is normal. KS is clinically homogenous and ultrastructurally heterogenous. REFERENCES 1. Kartagener M. Zur pathologie der bronchiectasien bei situs viscerum inversus. Beitrage Zur Klinic Der Tubekulose Und Spezifischen. 1933;83:489-501. 2. Oeri R. Bronchiectasis in situs inversus. Francfurter Zeitschrift fur Pathologie. 1901;3:393-8. 3. Siewert A. Uber einen fall von bronchiectasie bei einen patienten mit situs inversus viscerum. Berliner Klinische Wochenschrift. 1904;41:139-41. 4. Armengot M, Carda C, Basterra J. Disquinesias mucociliares. In: Gil Carcedo LM, Marco J, Medina J, Ortega P, Trinidad J, editors. Tratado de otorrinolaringología y cirugía de cabeza y cuello. Guadalajara: Proyectos Médicos; 1999. p. 638-46. 5. Afzelius B. Situs inversus and ciliary abnormalities. What is the connection? Int J Dev Biol. 1995;39:839-44. 6. Noone P, Leigh M, Sannuti A, Minnix S, Carson J, Hazucha M, et al. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med. 2004;169:457-9. 7. Munro N, Currie D, Lindsay K, Ryder T, Rutman A, Dewar A, et al. Fertility in men with primary ciliary dyskinesia presenting with respiratory infections. Thorax 1994;49:684-7. 8. Halbert S, Patton D, Zarutskie P, Soules M. Function and structure of cilia of the Fallopian tube of an infertile woman with Kartagener’ syndrome. Human Reprod. 1997;12:55-8. 9. Greenstone M, Rutman A, Dewar A, Mackay I, Cole P. Primary ciliary dyskinesia: cytological and clinical features. Q J Med. 1988;67:405-30. 10. Armengot M, Carda C, Basterra J. Axonema ciliar incompleto: ¿otra causa de discinesia ciliar primaria? Acta Otorrinolaringol Esp. 1998;49:57-9. 11. Afzelius B. A human syndrome caused by immotile cilia. Science. 1976;193:317-9. 12. Jorissen M, Bertrand B. Ciliary dyskinesia in the nose and paranasal sinuses. Acta Otorhinolaringol Belg. 1997;51:353-66. 13. Bounty O, Machillot D, Bellon G, Massonnet B, Dumontel C, Dutailly G, et al. Heterogeneite ultrastructurale dans le syndrome de dyskinesie ciliaire primitive. Ann Pediatr (Paris). 1990;37:432-6. Documento descargado de http://www.archbronconeumol.org el 19/11/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato. ARMENGOT CARCELLER M, ET AL. STUDY OF MUCOCILIARY TRANSPORT AND NASAL CILIARY ULTRASTRUCTURE IN PATIENTS WITH KARTAGENER’S SYNDROME 14. Herzon F, Murphy S. Normal ciliary ultrastructure in children with Kartagener’s syndrome. Ann Otol. 1983;89:81-3. 15. Greenstone M, Dewar A, Cole P. Ciliary dyskinesia with normal ultrastructure. Thorax. 1983;38:875-6. 16. Armengot M, Juan G, Barona R, Garin L, Basterra J. Immotile cilia syndrome: nasal mucociliary function and nasal ciliary abnormalities. Rhinology. 1994;32:109-11. 17. Armengot M, Ruiz G, Romero de Ávila C, Carda C, Basterra J. Utilidad diagnóstica del estudio del transporte mucociliar nasal con isótopos radiactivos en pacientes con infecciones respiratorias recidivantes. Rev Esp Med Nucl. 1998;17:21-6. 18. Armengot M, Basterra J, Garin L. Valores normales de aclaramiento mucociliar nasal. Comparación de diferentes técnicas y sustancias. Acta Otorrinolaring Esp. 1990;41:333-6. 19. Lurie M, Rennert G, Goldberg S, Rivlin J, Greenberg E, Katz I. Ciliary ultrastructure in primary ciliary dyskinesia and other chronic conditions: the relevance of microtubular abnormalities. Ultrastruct Pathology. 1992;16:547-53. 20. Nielsen M, Pedersen M, Christensen B, Mygind N. Blind quantitative electron microscopy of cilia from patients with primary ciliary dyskinesis and from normal subjects. Eur J Respir Dis. 1983;64 Suppl 127:19-30. 21. Rossman C. Primary ciliary dyskinesia: evaluation and management. Pediatr Pulmonol. 1998;5:36-50. 22. Benninger M. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology and pathophysiology. Otolaryngol Head Neck Surg. 2003;129:S1-S32. 23. Stannard W, Rutman A, Wallis C, O’Callaghan C. Central microtubular agenesis causing primary ciliary dyskinesia. Am J Respir Crit Care Med. 2004;169:634-7. 24. Carlen B, Lindberg S, Stenram U. Absence of nexin links as a possible cause of primary ciliary dyskinesia. Ultrastruct Pathol. 2003;27:123-6. 25. Chilvers M, Rutman A, O’Callaghan C. Ciliary beat pattern is associated with specific ultrastructural defects in primary ciliary dyskinesia. J Allergy Clin Immunol. 2003;112:518-24. 26. Brauer M, Viettro L. Aportes de la microscopía electrónica de transmisión al diagnóstico de la disquinesia ciliar. Rev Med Uruguay. 2003;19:140-8. 27. Sleigh M. Primary ciliary dyskinesia. Lancet. 1981;2:476. 28. Carson J, Collier A, Shih-Chin S. Acquired ciliary defects in nasal epithelium of children with acute viral upper respiratory infections. N Engl J Med. 1985;312:463-8. 29. Afzelius B, Camner P, Mossberg B. Acquired ciliary defects compared to those seen in the immotile cilia syndrome. Eur J Respir Dis. 1983;64 Suppl 127:5-10. 30. Pizzi S, Cazzato S, Bernardi F, Mantovani W, Cenacchi G. Clinicopathological evaluation of ciliary dyskinesia: diagnostic role of electron microscopy. Ultrastruct Pathol. 2003;27:243-52. 31. Armengot M, Basterra J, Castillo J, Marco V. Diagnóstico precoz del síndrome de inmotilidad ciliar mediante la técnica de la seroalbúmina marcada con Tc99m. Acta Otorrinolaring Esp. 1989;40: 137-40. 32. Armengot M, Basterra J. Nasal mucociliary function in the normal newborn. Int J Pediatr Otorhinolaryngol. 1991;22:109-13. Arch Bronconeumol. 2005;41(1):11-5 15